3 Jun 2019
Rachel Agass and Kate Loomes review the basic pathophysiology of this issue and explore some of the options for analgesia in the short term.

Recognition and treatment of pain is a daily occurrence for most ambulatory equine vets.
Identifying pain is simple in some instances – such as overt colic in a horse with a strangulating intestinal lesion, or non-weightbearing lameness observed in a horse with a limb fracture. However, pain can be more challenging to recognise in other cases.
The importance of not only recognising pain, but also being able to identify a response to analgesia, has led to the development of several equine pain scoring systems based on behavioural and physiological indices (Lindegaard et al, 2010; Dalla Costa et al, 2014; Gleerup et al, 2015).
The International Association for the Study of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (IASP, 2017).
The perception of physiological pain can alter the animal’s physiology and behaviour in an attempt to reduce or avoid damage, reduce the likelihood of recurrence and promote recovery (Molony and Kent, 1997).
Two main types of nociceptors exist – high-threshold mechanoreceptors, which respond to mechanical deformation; and polymodal nociceptors, which respond to cell signalling molecules, including proinflammatory mediators such as prostaglandins (Steeds, 2009).
Aδ and C afferent fibres transmit nociceptive information to the dorsal horn of the spinal cord, which ascends via spinothalamic and spinoreticular tracts to the somatosensory and prefrontal cortices, where conscious perception takes place (Steeds, 2009; Figure 1).
The neuroendocrine and metabolic responses to pain include – but are not limited to – increased sympathetic tone, increased stroke volume, heart rate and cardiac output, increased skeletal muscle tone, elevated adrenocorticotropic hormone and cortisol release, hyperglycaemia, increased protein metabolism and lipolysis, central hyperventilation, psychological effects, fibrinolysis, and platelet aggregation.
These reflex responses – induced by tissue damage and pain – are immediately protective for short-term survival, but can be deleterious if prolonged (Hellyer et al, 2007).
Neuronal plasticity refers to the potential for the response to stimuli to change over time through the process of neuronal sensitisation. Sensitisation may occur as a result of damage to the peripheral nervous system, which results in persistent noxious input from the periphery, leading to increased excitability of neurons in the dorsal horn of the spinal cord and the development of central sensitisation (Nishikawa and Nomoto, 2017).
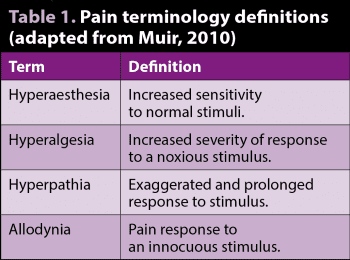
Peripheral and central sensitisation ultimately result in hyperaesthesia, hyperalgesia and allodynia, and these are characteristic symptoms of neuropathic pain (Table 1). Damage leading to neuropathic pain can result from a variety of insults, including trauma, vascular injury, endocrinopathy or infection (Hellyer et al, 2007).
Central and peripheral sensitisation have been demonstrated in the horse, and are frequently associated with poor outcome – emphasising the importance of appropriate pain management from the outset (Spadavecchia et al, 2004).
Pain has been broadly divided into three categories, based on underlying processes:
Nociceptive (physiological) pain – resulting from physiological nociceptive signalling. Usually sharp, localised and affecting a well-defined area. This is evoked by high-intensity stimuli, and is adaptive and brief. Examples include needle pricks and responses to hoof testers.
Inflammatory pain – this is evoked by high-intensity and low-intensity stimuli, secondary to tissue injury. Sensitisation often occurs. This pain is adaptive and reversible, and continues until inflammation decreases. Examples include lacerations, synovitis, surgery, and tendon and ligament injuries.
Neuropathic pain – maladaptive and persistent pain, evoked by high-intensity and low-intensity stimuli. Central sensitisation is sustained and pain can occur in the absence of continued noxious stimuli. Examples include nerve trauma and neuroma formation.
The aim of pain management should include the provision of pre-emptive analgesia and use of multimodal techniques.
Several factors must be considered when devising a therapeutic strategy, including the aetiology of the painful event, desired duration of therapy (acute versus chronic), desire for sedation, and potential side effects and toxicity (Clark and Clark, 1999). The evaluation of the effectiveness of the analgesia administered is also an important consideration.
Pre-emptive analgesia refers to analgesia administered prior to application of the noxious stimulus. In the field, this is not always possible as patients are often seen because they are perceived to already be in pain. In the elective surgical setting, however, pre-emptive analgesia is important and should be incorporated into the anaesthetic management of the patient.
Multimodal (balanced) analgesia refers to the concept of using two or more analgesic medications with different mechanisms of action, targeting different parts of the afferent nociceptive pathway, with the aim of producing additive or synergistic effects and reducing the side effects of any one substance (Lerche and Muir, 2009).
A balanced analgesia protocol has six main aims (Driessen, 2007):
Although many potential scenarios are encountered in practice, broadly speaking, pain management can be divided into acute and chronic management. Acute pain management may include treatment of acute colic or limb fractures in the field in the short term, perhaps to facilitate transportation to a hospital. Chronic pain management is usually indicated for ongoing lower grade painful conditions – for instance, medium-term to long-term management of ongoing orthopaedic issues, such as OA or laminitis.
Injectable medications are commonly administered in the short term, offering advantages over oral medications in terms of improved bioavailability and avoidance of first-pass metabolism.
NSAIDs exert their analgesic and anti-inflammatory action via inhibition of cyclooxygenase (COX) isoenzymes, which are mediators of the arachidonic acid cascade.
Over the past two decades, the focus has been on developing NSAIDs that are more selective for the COX-2 enzyme, ideally preserving the protective homeostatic function of COX-1 while inhibiting the proinflammatory and detrimental effects associated with COX-2 (Knych, 2017). However, it is now known COX-2 is also a constitutive enzyme in the eye, CNS and kidney, with COX-2 required for the healing of gastric ulcers (McFadzean and Love, 2018).
NSAIDs are the most frequently administered systemic analgesics in equine medicine. This class of analgesic also offers the benefit of a relatively wide variety of licensed products and routes of administration, as well as being familiar to owners (Taylor and Senior, 2018).
Clinicians may express a preference for the use of phenylbutazone in horses with orthopaedic pain and flunixin for horses with visceral pain, although this is not supported by evidence (Johnson et al, 1993).
Phenylbutazone has been shown to be effective in improving lameness scores and reducing heart rate in experimental models of lameness – for eight hours and six hours, respectively – using a single IV dose of 4.4mg/kg (Foreman et al, 2008). Flunixin meglumine (1.1mg/kg IV) and phenylbutazone (4.4mg/kg IV) were equally efficacious in improving experimentally induced foot pain when administered before, during and after treadmill exercise (Foreman et al, 2010).
Flunixin has been shown to provide superior postoperative analgesia in horses after surgical treatment of small intestinal strangulating lesions, compared to meloxicam (Naylor et al, 2014). Meloxicam offers preferential inhibition of the COX-2 enzyme, and has been shown to be effective in the control of postoperative pain and inflammation following orthopaedic surgery and acute synovitis (Walliser et al, 2015; de Grauw et al, 2009).
Ketoprofen exists as two forms of enantiomers that are rapidly absorbed and eliminated, have low volumes of distribution, and are highly protein-bound (Brink et al, 1998). Ketoprofen provides good to excellent analgesia for musculoskeletal pain; however, its efficacy in a model of acute synovitis was not superior to phenylbutazone (Owens et al, 1995).
While ketoprofen has a wider safety margin in horses compared to phenylbutazone and flunixin, its use is restricted to IV and IM administration (Goodrich and Nixon, 2006).
Firocoxib is a highly specific inhibitor of the COX-2 isoenzyme – and, therefore, COX-1 sparing – in contrast to phenylbutazone and other non-selective inhibitors of COX enzymes (Kvaternick et al, 2007). In a randomised prospective clinical study in horses recovering from surgical treatment of small intestinal strangulation, firocoxib and flunixin were equally effective in treating postoperative pain and firocoxib resulted in a greater reduction of a biomarker or endotoxaemia in these horses in the postoperative period (Ziegler et al, 2019).
Firocoxib is licensed for the treatment of pain associated with OA, and vedaprofen for the treatment of postoperative pain (McFadzean and Love, 2018). Firocoxib and vedaprofen treatments both resulted in reduction of lameness scores similar to that after treatment with phenylbutazone (Doucet et al, 2008; Koene et al, 2010).
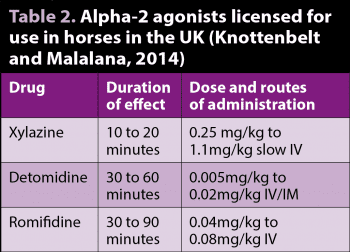
Alpha-2 adrenoreceptor agonists produce sedation, muscle relaxation and analgesia, and potentiate the effects of other sedative-hypnotic and anaesthetic drugs by activating alpha-2 receptors in the locus coeruleus and spinal cord (Muir, 2009).
The alpha-2 agonists vary in their potency, depending on their alpha 2-adrenoreceptor selectivity, and on their duration of effect. However, they all have profound effects on the cardiovascular system – including bradycardia, vasoconstriction followed by vasodilation, atrioventricular block, decreased coronary blood flow and reduced cardiac output (Sarazan et al, 1989). Careful consideration must, therefore, be given to patient selection and in cases where evidence exists of pre-existing cardiorespiratory compromise.
Xylazine, romifidine and detomidine are licensed for use in horses in the UK (Table 2). Xylazine has the shortest duration of effect, while detomidine and romifidine have longer durations of sedative and analgesic effect.
The visceral analgesia produced by xylazine at 1.1mg/kg IV is similar to that produced by flunixin meglumine, but the duration is shorter – making it useful for pain control during the initial evaluation of horses with colic. In a caecal balloon model of equine colic, xylazine (1.1mg/kg IV) produced the most pronounced visceral analgesia when compared with butorphanol (0.2 mg/kg IV), pethidine (1mg/kg IV) and pentazocine (0.99 mg/kg IV; Muir and Robertson, 1985).
Detomidine (20 ug/kg or 40ug/kg IV) provided superior analgesia compared to flunixin meglumine (1mg/kg IV) or butorphanol (0.1mg/kg IV). It must be used with care because it may conceal abdominal pain – and changes in heart rate and respiratory rate – for up to 90 minutes, which may confound the diagnosis (Jochle et al, 1989). An advantage is concurrent sedation allows for examination and transportation when severe abdominal pain remains unresponsive to other analgesics.
Other adverse effects seen with the administration of alpha-2 agonists include sweating, increased urine production (due to osmotic diuresis and antidiuretic hormone antagonism), upper airway obstruction in horses with upper airway disease, and reduced gastrointestinal motility (Zullian et al, 2011).
While new opioid receptors continue to emerge, most opiates are described and discussed on the basis of their activation of mu, kappa or delta opioid receptors (Muir, 2009). Full mu agonists include morphine, methadone and pethidine.
Opioids are somewhat underused in horses, probably due to conflicting evidence of their efficacy and potential side effects (Taylor and Senior, 2018). Despite successful clinical use, the use of opioids in horses is still controversial due to the potential sympathetic stimulation, arousal of the CNS, increased locomotor activity and reduction of gastrointestinal motility produced by these drugs (Lopes et al, 2016). It has been shown that by combining opioids with other drugs, such as the alpha-2 adrenergic agonists and phenothiazines (Love et al, 2012; Poller et al, 2013), the quality of sedation and analgesia can be improved while minimising the possible CNS excitation (Lopes et al, 2016).
Morphine is not licensed for use in horses in the UK, but is listed in the “Commission Regulation No 1950/2006 of 13 December 2006 establishing, in accordance with Directive 2001/82/EC of the European Parliament and of the Council on the Community code relating to veterinary medicinal products, a list of substances essential for the treatment of Equidae” for the purpose of analgesia.
While morphine is a potent and cost-effective analgesic in horses, decreased gastrointestinal motility, gastric distension and hyperphagia has been reported (Tessier et al, 2019). Oral bioavailability of morphine is poor, so IV, IM or epidural routes of administration are selected.
Full mu agonists are reported to exhibit dose-dependent increase in muscle tone and locomotor activity due to dopaminergic effects, and higher doses may cause euphoria/dysphoria or hyperexcitability.
Studies reporting unwanted side effects are generally from pain-free horses undergoing research, while the more “clinical” studies have shown beneficial effects (Mircica et al, 2003; Potter et al, 2016). The combination of an opioid with an alpha-2 agonist helps prevent the excitatory effects and potentiates the sedative action of the latter.
Methadone is licensed for use in cats and dogs in the UK. In experimental horses, an antinociceptive effect to mechanical, thermal and electrical stimuli has been demonstrated (Lopes et al, 2016). However, evidence relating to the analgesic effect of methadone on animals experiencing clinical pain is limited.
Buprenorphine is a partial mu agonist, demonstrating excellent receptor affinity, but only partial agonist activity once bound. IV administration of buprenorphine provided superior antinociception to thermal and mechanical stimuli, compared to butorphanol (Love et al, 2012). These experimental findings were confirmed in a clinical study where buprenorphine provided more effective postoperative analgesia than butorphanol after elective surgical procedures performed under general anaesthesia without detrimental effects (Taylor et al, 2016).
Pharmacokinetic studies have demonstrated analgesic concentrations present in plasma 30 minutes after IV administration of 0.005mg/kg, although higher doses may be required for IM use (Davis et al, 2007).
CNS excitement and decreased gastrointestinal motility have been noted, particularly with IV use. Sublingual bioavailability has been shown to be good, with similar plasma concentrations to IV use in one study (Messenger et al, 2011).
Butorphanol is a mu antagonist and kappa receptor agonist. Studies on visceral pain models mostly show a dose-dependent analgesic effect of short to intermediate duration (15 minutes to 90 minutes; Kalpravidh et al, 1984), while somatic pain studies have shown conflicting results. Butorphanol had no effect on the nociceptive withdrawal reflex using threshold, suprathreshold and repeated subthreshold electrical stimuli in conscious horses (Spadavecchia et al, 2007).
Previously reported analgesic effects of butorphanol have been associated with doses far higher than those used for sedation in horses, resulting in adverse effects such as ataxia and restlessness (Kalpravidh et al, 1984).
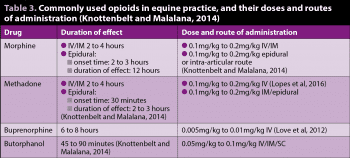
Table 3 lists the doses and routes of administration for opioids commonly used in equine practice.
Local anaesthetics are the only agents that completely block sensation; therefore, locoregional anaesthesia adds significantly to multimodal analgesia (Taylor and Senior, 2018).
Their mechanism of action is via sodium ion channel blockade – therefore preventing action potential propagation in Aδ and C fibres, resulting in local desensitisation.
Mepivacaine is licensed in the UK for use in horses via infiltration, perineural, intra-articular and epidural routes.
Lidocaine, combined with epinephrine, is licensed for regional nerve block and infiltration anaesthesia.
Procaine, combined with epinephrine, is licensed for perineural anaesthesia, although concerns exist regarding white hair growth at injection sites in horses.
Intratesticular administration of lidocaine has been shown to reduce the number of movements – and, therefore, additional ketamine requirements – in horses undergoing castration under injectable anaesthesia (Portier et al, 2009). Perineural administration of local anaesthetic or locoregional techniques, involving SC infiltration, are useful to desensitise areas of skin for wound repair, for example.
One limitation of local anaesthetics is their reduced performance in infected tissues; this is due to the altered pH of the tissue affecting the fraction of unionised drug (Ueno et al, 2008). In this instance, a nerve block at a site remote from the infected tissue may be beneficial.
Perineural administration of local anaesthetic may have limited applications in the field due to the relatively short duration of effect. However, the use of local anaesthesia may be particularly useful in certain situations, such as to facilitate the transport of a painful laminitic case to a hospital facility. Generally, desensitisation of orthopaedic injuries is not recommended as further tissue injury may occur with increased loading of injured structures.
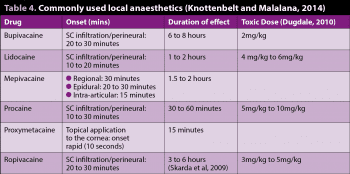
Local anaesthetics vary in terms of their pharmacokinetic properties, which influence the speed of onset and duration of effect (Table 4).
Most appropriately applied to orthopaedic patients, external coaptation or support can contribute significantly to comfort.
For example, with limb fractures, while a degree of pain may be protective of further injury, the sensation of limb instability is a source of significant anxiety and distress. Application of appropriate external coaptation can relieve some of the associated anxiety and facilitate transportation, while helping limit further tissue injury. Specific recommendations for external coaptation of fractures is not the focus of this article.
Similarly, with respect to laminitis cases, provision of mechanical support for the foot aims to counteract the distractive forces; the upwards ground reaction force and the caudal “pull” of the deep digital flexor tendon (DDFT), causing distraction of the distal phalanx from the dorsal laminae. This is generally achieved using some form of sole support and heel elevation, resulting in reduction in strain in the DDFT. Support can result in significant improvement in comfort and limit progression of structural changes within the foot.