27 Mar 2018
Andy Durham outlines the two-step process for investigating causes of pruritus in horses – including how decide there is an allergy at all.
Pruritus is a common clinical sign in horses and generally indicates either parasitism or allergy (Table 1). Both age and duration of exposure to the suspected allergen are important considerations in the context of allergy.
A period of prior exposure and sensitisation is a prerequisite for development of allergy, and foals and young horses are, therefore, rarely affected. Contrary to popular belief, a suspected allergen is highly unlikely to be something any horse has just encountered for the first time.
Whatever the cause of skin allergy, typical clinical signs comprise pruritus and/or urticaria, with secondary self-inflicted hair loss and skin trauma. Parasitism may also present as pruritus, and sometimes local urticarial reactions can also be seen to arthropod bites.
Distribution of skin lesions may offer a clue regarding causation. Insect bite hypersensitivity typically has a dorsal distribution, with the mane and tail especially affected – although ventral, and even generalised, pruritus may occur. Conversely, atopy cases and drug reactions usually show generalised pruritus or urticaria, although localised signs may sometimes occur.
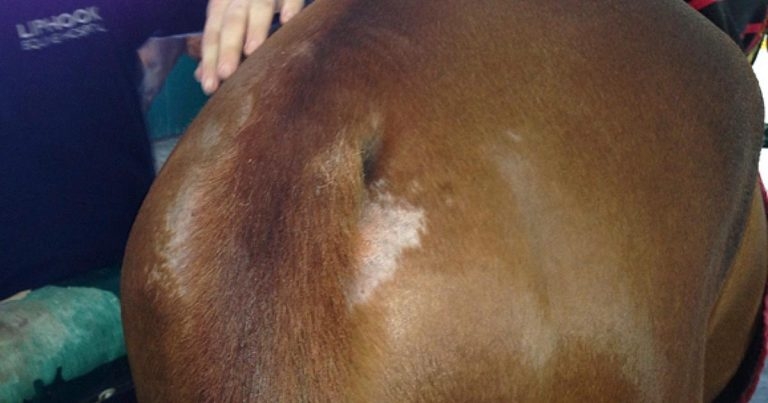
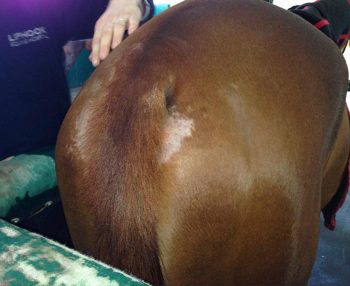
Localised urticarial reactions raise the possibility of contact allergy or, perhaps more likely, contact irritancy. Oxyuris equi infestation results in perineal pruritus and self-trauma, although, notably, usually not affecting the dorsal surface of the tail as seen in sweet itch cases (Figure 1). Chorioptes infestation is generally limited to the lower limbs and Sarcoptes often to the mane, tail and face.
Other free-living mites tend to irritate the horse in areas such as the lower limbs and face.
Investigation of a suspected allergy is a two-step process. Step one is to decide whether the horse has an allergy, and step two is to attempt to define what allergen(s) is implicated in the problem.
It is important to realise no specific diagnostic tests are available that might tell you a horse has an allergy (step one). To make a diagnosis of an allergy in a horse, a clinical process of elimination is required, largely aimed at ruling out parasitic causes.
If or when it is decided the horse genuinely has an allergic problem,
further tests (step two) can be used in attempts to identify the cause. Little to no evidence supports the use of serum
allergy tests in horses, despite their ease and convenience.
Intradermal testing remains the preferred method for identifying causes of atopic allergy in horses, with patch testing and feed exclusion trials also part of the overall investigative process. The author has been performing intradermal testing in horses for more than 20 years, and practitioners wishing to use this simple and informative technique may contact him for further details of technique and availability.
Essentially, three outcomes are possible from intradermal testing. Firstly, it is possible nothing useful is found, although, thankfully, this is not a common outcome if parasites and other differential diagnoses (Table 1) have first been investigated thoroughly. Secondly, an allergen(s) may be identified that can then be avoided or eliminated from the horse’s environment (for example, wool, house dust, cat hair or mites). Thirdly, environmental allergens may be identified that cannot be avoided (for example, pollens or fungal spores), and allergen-specific immunotherapy (ASIT) might then be considered for such cases (see later).
No well-described publications report food allergies in horses, although they may well exist. Feed exclusion trials are the technique of choice to establish the diagnosis, although they are remarkably inconvenient as they must involve the strict elimination of all oral intake other than water and one basic feed type.
Grass cubes, alfalfa, timothy grass or rye grass haylage are possible, but box confinement is essential to prevent grazing multiple plant types in pasture. The length of a feed exclusion trial probably needs to be at least four to six weeks or until the time clinical signs resolve. Trial exposure of individual feedstuffs would hopefully then establish the original cause.
ASIT solutions are commercially available for a wide range of potential allergens, and are selected on the basis of intradermal test results. Protocols usually comprise initial frequent injections of increasingly concentrated allergen solutions, leading to less frequent (every one to two weeks) maintenance therapy that should continue for at least two years, and perhaps for life.

Prerequisites for successful ASIT must logically include both identification of the specific allergen concerned, and also access to pure preparations of the specific allergen for re-presentation to the horse. Although these prerequisites are satisfied reasonably well in many atopic dermatitis cases, they are, thus far, not satisfied in the case of sweet itch.
It is important to bear in mind what, specifically and exactly, the allergen for inclusion in ASIT in clinical cases is. For example, the diagnosis of an allergy to oak tree pollen may be followed up by use of ASIT containing oak tree pollen antigen in a sterile solution. In contrast, following a diagnosis of insect bite hypersensitivity, the allergen(s) is not so easy to specify.
Several studies have identified a range of distinct insect salivary proteins relevant to causation of sweet itch, and these proteins may differ from region to region and horse to horse (Wilson et al, 2008; Hellberg et al, 2006; Ferroglio et al, 2006). One study detected 10 specific Culicoides salivary proteins that sweet itch cases reacted to, with typically three to four specific and different allergenic proteins per horse (Hellberg et al, 2006).
Additionally, it might not be salivary proteins exclusively that lead to sensitisation and sweet itch. Other proteins, such as those found in the eye, abdomen and thorax of the flies, may well be relevant in a few cases – perhaps resulting from sensitisation when flies are crushed into the skin when a horse rubs (Wilson et al, 2001).

Unfortunately, it seems we are unable to identify the specific individual allergenic proteins in suspected clinical sweet itch cases, and, therefore, application of specific targeted ASIT is theoretically compromised.
All that is available, both for intradermal testing and for ASIT, are crude whole fly extracts, rather than individual pure protein solutions. Hopefully, in time, specific proteins will be included in a panel of sweet itch salivary and non-salivary antigens for diagnostic and therapeutic purposes in sweet itch cases.
Clearly, this issue is less of a concern where more specific allergen identification has been made, such as pollens or animal danders, which are available. As use of ASIT may require persistence for up to a year before benefits are seen, it is important to plan to begin immunotherapy well in advance of expected onset of signs in cases of seasonal allergy.
ASIT is an attractive technique when avoidance is not possible or practical. It is always very difficult to know, with certainty, the effectiveness of various ASIT protocols and to separate placebo or natural resolution from true efficacy. For example, in one study of attempted ASIT
for sweet itch, 50% of owners of horses in the blinded placebo group felt their horses had improved on “treatment” (Barbet et al, 1990) in a nice demonstration of the power of the placebo effect. In that six-month study, no significant benefit of ASIT for sweet itch was seen, although some
might argue this was too short a period for proper evaluation of results.
Other studies with larger numbers of cases studying a variety of allergens over a longer time period have indicated far better efficacy of ASIT in horses. A follow-up of 32 horses with urticaria and/or pruritus found owner-reported improvement of clinical signs in 84% of cases (Stepnik et al, 2012), although further scrutiny of these revealed ASIT had allowed total discontinuation of all other therapies in 59% of the 32 cases, with a further 9% able to discontinue glucocorticoids at least.
Interestingly, of 15 horses where ASIT had been discontinued due to resolution of clinical signs, five experienced recurrence of clinical signs. Three of these were placed back on to ASIT and signs resolved again in all three.

The parasitic life cycle and sources of nutrition are important determinants of the medical approach to elimination of parasites. For example, deep-living dermal parasites, such as Pelodera, Onchocerca, Habronema, Sarcoptes and blood-sucking parasites, such as sucking lice (Haematopinus asini), are likely to be eliminated by systemic medication with ivermectin or moxidectin (not licensed for this purpose).
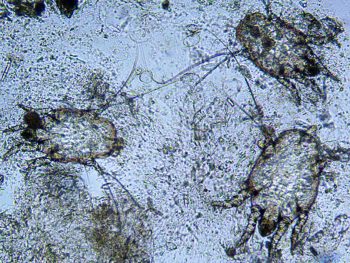
More superficially living ectoparasites feeding on surface debris, such as the more common biting louse (known as either Bovicola equi or Damalinia equi; Figure 2) or Chorioptes mites (Figure 3)
may respond less well to systemic treatments, and topical applications of
lime sulphur, selenide and ectoparasiticides, such as permethrins or fipronil, are usually preferred.
As with all parasitic treatments, reinfestation is a major concern, and repeat treatments are generally required. This is especially problematic when treating chorioptic mange, which is extremely difficult to cure permanently.
Repeat treatments at two to three-week intervals may successfully kill newly hatched chorioptic mites, although reinfestation from the environment (where the mites may live for a few days at least), or from other in-contact horses (which may or may not be showing clinical signs), are both major
Oxyuris equi (Figure 4) continues to cause frustration among horse owners and veterinary surgeons, and generally proves difficult to eliminate, owing to its colonic location that avoids therapeutic concentrations of most anthelmintics.
No anthelmintics have, thus far, been found to be consistently fully efficacious against Oxyuris adults or larvae. For example, in one study, the efficacy of a standard dose of ivermectin and a double dose of pyrantel were 96% and 91%, respectively, against Oxyuris adults and 99% (both drugs) against Oxyuris larvae, suggesting the presence of some resistance to these two drugs among adult worms.
However, although the larval kill rate might sound impressive, half of the treated horses still had more than 1,000 surviving larvae post-treatment (Reinemeyer et al, 2010). In an analogous process to faecal collection from pasture for control of nematodes, it is crucial daily perineal washes are performed in Oxyuris-infected horses to remove eggs and eventually break the life cycle of reinfection, as total reliance on anthelmintics is likely to lead to overuse and lack of efficacy.