28 Jan 2020
Kate Loomes BVSc(hons), MSc, CertAVP(EP), CertAVP(VA), CertAVP(EM), DipECVAA, MRCVS in the first of a two-part article, looks at the intraoperative issues that can arise while horses are under anaesthesia.
Perianaesthetic mortality rates remain higher in horses compared to our other commonly anaesthetised veterinary species – dogs (0.05%), cats (0.11%) and rabbits (0.73%; Brodbelt et al, 2008).
In 2002, a large multicentre study in the UK reported a perianaesthetic mortality rate of 0.9% in systemically healthy horses undergoing a variety of surgical procedures (Johnston et al, 2002).
While significant anatomical and physiological differences may exist between our veterinary species, it is evident equine anaesthesia involves considerable risk.
Complications may arise at any time during the perianaesthetic period – from the preparation for induction of general anaesthesia, through to the postoperative period.
Pharmacodynamic and pharmacokinetic interaction and effect of the anaesthetic agents, specific disease processes, physiological variation, nature of the surgical intervention, duration of the anaesthetic period, positioning of the horse, temperament of the horse and human error can all play a role in the development of anaesthetic complications.
Awareness of some of the more common intraoperative complications that may be encountered may aid prompt recognition and initiation of treatment measures.
Hypotension is a common complication encountered during equine inhalational anaesthesia, and usually results from the negative effects of inhalational agents on myocardial contractility and reduction in vascular resistance, resulting in vasodilation.
Hypotension can also be a consequence of reduced venous return (seen in dorsal recumbency in large horses [Figure 1], or in the presence of abdominal distension or during positive pressure ventilation), bradycardia, tachycardia, severe blood loss, hypovolaemia or pre-existing dehydration.
While the definition of hypotension may differ between literature sources, maintaining a mean arterial blood pressure (MAP) greater than 70mmHg has been associated with a reduction in the severity of post-anaesthetic myopathy in halothane-anaesthetised horses (Duke et al, 2006).
Muscle blood flow may be better preserved during isoflurane anaesthesia compared to halothane, even during periods of hypotension; however, both agents produce a dose-dependent reduction in skeletal muscle blood flow at both arterial and microvascular levels (Raisis, 2005).
Hypotension may also be linked to detrimental effects on other organ systems due to compromised perfusion – and for these reasons, aiming to maintain a MAP of 70mmHg or above is recommended.
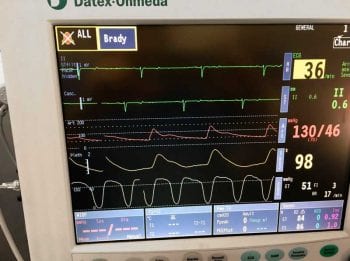
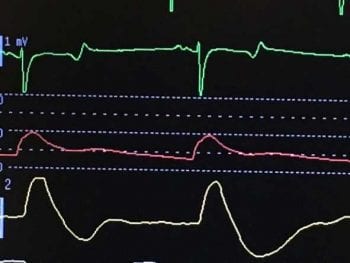
Since arterial blood pressure results from the relationship between blood volume and the relative blood volume capacity, several contributing factors can be involved in the development of hypotension. Arterial blood pressure can be measured using non-invasive or invasive techniques (Figures 2 and 3).
Considering the relatively frequent incidence of hypotension in anaesthetised horses and the potential consequences, invasive blood pressure monitoring is employed routinely during general anaesthesia in most equine facilities.
Manual assessment of pulse quality using digital palpation at the peripheral sites (transverse facial, mandibular, dorsal metatarsal arteries) provides an indication of the difference between systolic and diastolic blood pressure, and the ease with which the pulse can be occluded may provide an indication of the MAP.
While this technique remains subjective, it is a useful skill to practise while directly measuring arterial blood pressure to improve interpretation of the palpable pulse character.
The shape of the arterial blood pressure waveform can provide useful information, and will vary in appearance depending on the relationship between diastolic, mean and systolic blood pressure values, and vessel tone (Figures 4 and 5).

Volatile anaesthetic agents, isoflurane and sevoflurane cause suppression of vasomotor tone due to their effect on the CNS. The dose-dependent reduction in systemic vascular resistance can result in hypotension.
For reasons not entirely known, horses appear to be particularly sensitive to the negative myocardial and vasodilatory effects of inhalational agents – meaning even healthy horses that are lightly anaesthetised and moving can be hypotensive (Wagner, 2009).
Anaesthetic depth should be re-evaluated and the delivered concentration of volatile agent decreased, where appropriate. However, hypotension commonly develops at appropriate depths of anaesthesia – and while reduction of delivered volatile agent alone is not always an option, the integration of IV anaesthetic agents (ketamine, lidocaine) or sedatives (alpha-2 agonists), and employment of a partial IV anaesthetic technique, may afford a volatile anaesthetic-sparing advantage allowing the safe reduction in delivered volatile agent, while maintaining an appropriate anaesthetic depth.
IV fluid administration (Figure 6) aims to maintain an adequate circulating vascular volume to preserve venous return in the face of volatile anaesthetic agent-related vasodilation.
The type and rate at which IV crystalloid fluids are administered can alter transvascular fluid dynamics and may contribute to tissue oedema if rates are excessive.
Administration of a balanced electrolyte crystalloid fluid, such as Hartmann’s solution, is routine practice intraoperatively. Dehydrated horses have a fluid deficit that affects all body compartments, while hypovolaemic horses have a vascular volume deficit. It is possible for dehydration and hypovolaemia to co-exist.
Crystalloid fluids – such as Hartmann’s solution – can be used to increase the circulating vascular volume; however, due to the size of the average horse, administering a sufficient volume in a short time requires placement of additional venous cannulas and pressurised fluid-bag sleeves to increase the rate of administration.
Additionally, crystalloid fluids remain in the vascular space for a relatively short time, with between 60% and 80% of the administered volume being redistributed out of the vascular space and into the interstitium within 30 minutes to 60 minutes – making the vascular expansion effect short-lived.
A low diastolic arterial pressure (lower than 40mmHg) may indicate hypovolaemia either due to circulating volume loss, or relative hypovolaemia caused by excessive vasodilation.
Colloid solutions may exert a greater volume-expansion effect as they contain large molecules that are unable to cross the intact vascular endothelium. This property allows colloid fluids to impart an effective oncotic pressure across the endothelium, which acts to draw fluid into the vascular space. The resulting vascular expansion exceeds the volume of colloid fluid administered.
Different types of colloid solutions exert different degrees of oncotic pressure for different lengths of time; however, several of the large molecular starch solutions have been withdrawn from use in human medicine. Modified gelatin solutions are available and exert a colloidal effect.
A combination of crystalloid and colloid fluids can be used to increase the vascular volume. In cases of severe haemorrhage, whole blood should be used to replace some of the volume lost and maintain oxygen-carrying capability.
Dobutamine is a positive inotrope with primarily cardiac beta-1 adrenergic receptor agonist effects. It is administered via an IV infusion at rates between 0.2µg/kg-1/min-1 to 4µg/kg-1/min-1, and results in an increased force of myocardial contraction.
Some activation of cardiac and vascular beta-2 adrenoreceptors may also occur, resulting in vasodilation (Raisis, 2005).
Dobutamine should be carefully titrated to effect and may be less effective in hypovolaemic horses if poor venous return and poor diastolic pressure are present. In these cases, tachycardia may result from dobutamine administration, which tends to impede cardiac filling and fail to improve cardiac contractility or arterial blood pressure.
Dobutamine administration combined with a crystalloid fluid bolus (20ml/kg-1) in isoflurane-anaesthetised hypotensive horses has been shown to improve femoral arterial blood flow, but no impact was observed on cardiac index with this combination (Loughran et al, 2017).
Ephedrine is a synthetic, non-catecholamine, sympathomimetic amine that has direct agonist effects at alpha-adrenergic and beta-adrenergic receptors, and has indirect effects mediated via the release of norepinephrine from sympathetic nerve endings (Daunt, 1990; Egger et al, 2009).
Ephedrine may be described as an inoconstrictor, as beta-1 adrenoreceptor activity manifests as positive inotropy, while the alpha-adrenoreceptor activity manifests as peripheral vasoconstriction (Dugdale, 2010).
Ephedrine can be given as a single injection (0.03mg/kg-1 to 0.05mg/kg-1 IV) or incremental smaller boluses (0.02mg/kg-1 IV); administration via infusion (0.02mg kg-1/min-1 IV) has also been described (Fantoni et al, 2013).
Ephedrine has been shown to increase MAP by increasing cardiac index and systemic vascular resistance, and results in better tissue oxygenation when compared to phenylephrine infusion (Fantoni et al, 2013).
Administration of higher doses (0.2mg/kg-1 IV) has been associated with the development of cardiac arrhythmias (Lee et al, 2002). The first dose tends to have the greatest effect as repeated doses may not allow time for norepinephrine replenishment, leading to the phenomenon of tachyphylaxis (reduced effectiveness with subsequent dose; Dugdale, 2010).
Phenylephrine acts at alpha-1 adrenergic receptors, causing vasoconstriction. In situations where hypotension does not respond to minimisation of volatile agent delivery, administration of fluid therapy or dobutamine/other vasopressor administration, phenylephrine administration combined with IV fluid therapy may act to improve the relative hypovolaemia and hypotension caused by systemic vasodilation.
Phenylephrine does, however, act to increase cardiac afterload, increase myocardial oxygen demand, decrease cardiac index, and cause vasoconstriction of the splanchnic and peripheral vasculature, which can have detrimental effects on gastrointestinal and muscle perfusion (Dugdale et al, 2007).
Phenylephrine can be infused IV to effect at rates between 0.5µg/kg-1/min-1 to 1µg/kg-1/min-1, but should be reserved for situations where excessive vasodilation is suspected – such as in endotoxaemic crisis or when other treatment techniques have failed to improve MAP to greater than between 60mmHg and 70 mmHg.
Hypertension is uncommon in anaesthetised horses. Causes may include increased sympathetic stimulation – such as an increase in nociception during a surgical procedure where inadequate analgesia, or employment of local anaesthetic techniques, are present.
Neurectomies can elicit a sympathetic response, which is usually short-lived and responsive to additional analgesia. An inadequate plane of anaesthesia may result in an excessively light plane of anaesthesia and an elevation in arterial blood pressure may be seen before other signs, such as limb or head movement. More rarely, conditions such as phaeochromocytoma can result in a persistent, and worsening, tachycardia and hypertension.
Hypertension seen in the early phase of anaesthesia may be attributed to a pre-existing condition, such as severe abdominal pain in horses anaesthetised for exploratory laparotomy due to colic, or in a particularly agitated or anxious horse.
Once an adequate plane of anaesthesia has been reached, the reduction in systemic vascular resistance and vasodilation caused by the volatile agents usually acts to reduce MAP to normotensive values.
Hypoxaemia is the poor oxygenation of blood – and hypoxia is the poor oxygenation of tissue.
The definition of hypoxaemia is an arterial oxygen tension (PaO2) of less than 60mmHg.
A small proportion of blood oxygen content is dissolved in plasma, but the vast majority of oxygen in blood is bound to haemoglobin.
Several factors affect the unloading of oxygen from haemoglobin to tissue, and the relationship between haemoglobin oxygen saturation and the arterial partial pressure of oxygen is related to the changing shape of the haemoglobin molecule.
The sigmoid shape of the oxygen haemoglobin dissociation curve indicates that haemoglobin oxygen saturation starts to fall rapidly once PaO2 declines below 80mmHg – and the reduction is very rapid once PaO2 falls lower than 60mmHg.
Identifying and treating mild hypoxaemia (PaO2 between 80mmHg and 90mmHg) can be beneficial, rather than waiting for severe hypoxaemia to develop when PaO2 is less than 60mmHg and haemoglobin oxygen saturation is likely to be less than 90%.
Pulse oximetry is a non-invasive method of continuously measuring arterial haemoglobin oxygen saturation. Factors such as vasoconstriction, tissue thickness, tissue contact and movement can affect the ability of the probe to accurately detect the pulsatile arterial blood flow. Arterial blood gas analysis is an accurate method of measuring arterial oxygen tension.
If hypoxaemia has been identified, it can be useful to consider the journey oxygen takes from the cylinder to the blood, to try to establish the cause of hypoxaemia:
Life-threatening consequences of hypoxaemia include cerebral hypoxia and respiratory arrest, myocardial hypoxia, and fatal cardiac arrhythmias.
Peripheral tissue hypoxia results in anaerobic metabolism and lactic acidosis, which causes global cellular injury.
An arterial oxygen tension of less than 80mmHg – and anaesthetic duration greater than two hours – increased the incidence of surgical site infection post-emergency exploratory laparotomy ninefold (Costa-Farré et al, 2014).
During inhalational anaesthesia, respiratory depression is common and often necessitates the use of controlled mechanical ventilation in horses (Figures 7 and 8).
Ventilator settings can be adjusted to ensure a tidal volume of 10ml/kg-1 to 15ml/kg-1, a respiratory rate of between six breaths and eight breaths per minute-1, an inspiratory:expiratory ratio of 1:2 or 1:3, and an inspiratory time of two seconds to three seconds is achieved without exceeding a peak inspiratory pressure of 20cm to 26cm H2O (Kerr and McDonnell, 2009).
Ventilator design varies, but the basic components that contribute to minute ventilation are consistent.
The provision of positive airway pressure acts to reduce venous return and can have a detrimental effect on the cardiovascular system, resulting in a reduction in cardiac output and MAP. These effects must be considered when adjusting ventilator settings.
Aerosolised beta-2 agonist salbutamol has been shown to improve PaO2 in hypoxaemic horses during anaesthesia (Robertson and Bailey, 2002).
Delivery of 2µg/kg-1 aerosolised salbutamol was performed via the endotracheal tube during the inspiratory phase of respiration and was not associated with detrimental side effects. Conversely, IV clenbuterol improves PaO2, but is associated with profuse sweating and tachycardia (Keegan et al, 1991).
Therefore, aerosolised salbutamol is the recommended route of administration of a beta-2 agonist in the treatment of hypoxaemia during general anaesthesia.
The reopening of closed alveoli is described as lung recruitment and can be effective in improving gas exchange. This can be performed by applying positive end expiratory pressure (PEEP).

PEEP is the positive pressure that is maintained in the breathing system by the mechanical ventilator at the end of expiration. The technique acts to prevent alveolar collapse and, therefore, improve ventilation perfusion matching.
Step-wise titration of PEEP values – from 5cm to 15cm H2O – has been shown to improve gas exchange during inhalation anaesthesia in horses (Ambrósio et al, 2013); however, since the application of PEEP results in continued generation of positive pressure in the thorax, it can have a detrimental effect on cardiovascular parameters.
The effect of PEEP on MAP must be monitored and additional cardiovascular support may be required. The generation of high airway pressures has been shown to have a linear pressure-dependent decrease in cardiac index and MAP, which was also associated with a reduction in intestinal microperfusion (Hopster et al, 2017).
In recovery, the change in body position from dorsal to lateral, then to sternal recumbency improves oxygenation by restoring the normal anatomical dynamic between pulmonary blood flow and aerated regions of lung tissue.
A demand valve can be used to deliver 10L/min-1 to 15L/min-1 oxygen in spontaneously breathing horses, and has been shown to improve PaO2.
Hypocapnia is generally associated with hyperventilation and may be seen with inadequate depths of general anaesthesia or in response to nociception.
Hypocapnia is rare during equine general anaesthesia, as most volatile anaesthetic agents cause respiratory depression.
Hypercapnia is an arterial carbon dioxide tension (PaCO2) of greater than 45mmHg, with moderate to severe hypercapnia occurring when PaCO2 exceeds 70 mmHg (Kerr and McDonnell, 2009).
General anaesthesia depresses the response of central respiratory centres to rising arterial carbon dioxide, and the normal compensatory increases in tidal volume and respiratory rate are blunted – resulting in hypercapnia.
Mild to moderate increases in PaCO2 have a stimulatory effect on the sympathetic nervous system, and act to increase cardiac output, decrease systemic vascular resistance, increase tissue perfusion, and increase tissue oxygenation and oxygen availability.
However, when PaCO2 rises to greater than 70mmHg, respiratory acidosis ensues – which causes hyperkalaemia, myocardial depression, catecholamine release, tachycardia, cardiac arrhythmias and CNS depression (Kerr and McDonnell, 2009).
Depending on pulmonary ventilation-perfusion dynamics, the difference between end-tidal carbon dioxide tension (ETCO2) and PaCO2 can vary from between 10mmHg to 15mmHg in a normal horse, to greater than 30mmHg in horses where extensive ventilation-perfusion mismatching is present.
It can, therefore, sometimes be difficult to predict PaCO2 based on ETCO2; blood gas analysis is the only reliable method of measuring PaCO2 during general anaesthesia.
In spontaneously breathing horses, the provision of controlled mechanical ventilation should act to correct hypercapnia in most cases.
Adjustment of ventilator settings should be tailored to improving end-tidal carbon dioxide tension in hypercapnic horses already receiving mechanical ventilation.
In horses where increased abdominal distension is present – such as during colic surgery – the tidal volume delivered may be limited by the intrathoracic pressure generated. In these cases, the respiratory frequency can be increased to try to restore normocapnia.
If the ventilator settings appear to be appropriate and hypercapnia continues, then investigation into machine malfunction should be carried out. Rebreathing of expired carbon dioxide can occur when soda lime (or other carbon dioxide absorbents) is exhausted or when one-way valves are malfunctioning. In these cases, the capnograph trace will fail to return to zero, which indicates the rebreathing of carbon dioxide – therefore contributing to an overall elevated end-tidal carbon dioxide tension.
The initial evaluation of an abnormal cardiac rhythm must consider the degree of haemodynamic consequence associated with it, and the potential for progression for further myocardial destabilisation and fibrillation.
Pathological arrhythmias tend to develop in the presence of factors such as high sympathetic tone, pyrexia, endotoxaemia, sepsis, gastrointestinal disease, electrolyte imbalance, acidosis, hypoxaemia and severe pulmonary disease.
Sinus bradycardia can be caused by alpha-2 agonists, inhalational anaesthetic agents, ocular manipulation, traction on gastrointestinal organs, hypoxia, hyperkalaemia or hypothermia. Since heart rate is a determinant of cardiac output, bradycardia may reduce cardiac output and cause hypotension.
In symptomatic cases where haemodynamic consequences are present, the bradycardia should be treated by reversal of alpha-2 agonists if these have been used.
Atipamezole (50µg/kg-1) has been shown to antagonise detomidine-induced bradycardia in standing horses (Zeiler, 2015).
Most bradyarrhythmias are caused by high vagal tone and respond to the administration of anticholinergics, but be aware anticholinergic agents are associated with reduced gastrointestinal motility and ileus in horses.
Glycopyrrolate (0.005 mg/kg-1 to 0.01mg/kg-1 IV) has little CNS effect, tends to cause a steady increase in heart rate and has a shorter duration of effect than atropine (0.005mg/kg-1 to 0.02mg/kg-1 IV).
Dobutamine (IV infusion 1µg/kg-1/min-1 to 4µg/kg-1/min-1) can be used to improve blood pressure and increase heart rate.
Epinephrine is usually reserved for acute severe bradycardia and sinus arrest.
Hyperkalaemia can be associated with cardiac arrhythmias, particularly if serum potassium is greater than 6mmol/L-1. ECG changes seen include:

Administration of 23% calcium gluconate (0.2ml/kg-1 to 0.4ml/kg-1 IV) in 5% dextrose (6ml/kg-1 IV) helps to stabilise the myocardium and counteract the cellular effects of potassium.
Administration of regular insulin (0.1IU/kg-1 IV) and dextrose (5ml/kg-1 to 10ml/kg-1 IV) drives potassium intracellularly.
If metabolic acidosis is present, sodium bicarbonate (1milliequivalent/kg-1 to 2milliequivalents/kg-1 IV slowly over 15 minutes) can be given to correct the pH and drive potassium into cells (Schwarzwald et al, 2009).
Be aware sodium bicarbonate administration will liberate carbon dioxide, so ensure mechanical ventilation is available, if required, to avoid producing a respiratory acidosis that will worsen the situation.
Sinus tachycardia can be caused by excitement (inadequate anaesthetic depth), nociception, hypovolaemia, hypotension, endotoxaemia, anaemia and, more rarely, phaeochromocytoma. The underlying source of the tachycardia should be identified and treatment tailored to this.
Atrial premature complexes (APC) arise in the atria, but not from the sinoatrial node, and the premature P wave often looks different from the “normal” P wave.
Some premature P waves are conducted and others are not, depending on the timing of arrival at the atrioventricular (AV) node.
Atrial arrhythmias can develop due to high sympathetic tone, anaemia, hypoxia, drugs (catecholamine, anaesthetic agents), sepsis, pyrexia, systemic inflammation, atrial myocardial inflammation, fibrosis or ischaemia.
Isolated and occasional APCs often have little effect on haemodynamic stability, while frequent and sustained APCs can progress to atrial fibrillation (AF).
The rapid atrial impulses are not all conducted by the AV node, so the atrial rate is slower than the ventricular rate and the ventricular rate is usually irregularly irregular in horses with AF.
Rapid atrial rates can compromise cardiac filling, and have a detrimental effect on cardiac output and MAP. Where possible, atrial arrhythmias should be treated prior to general anaesthesia, but in horses that develop atrial arrhythmias during general anaesthesia that compromise arterial blood pressure, efforts should be directed at maintaining normotension and adequate circulating volume.
Ventricular complexes arise distal to the AV node; therefore, the QRS morphology is abnormal, and is often described as wide and bizarre. Ventricular premature complexes (VPCs) that are occasional and isolated often have little effect on haemodynamic stability – and while treatment is often not warranted, the occurrence should be closely monitored.
The initial bolus can be followed by a continuous IV infusion of 0.05mg/kg-1/min-1 (Schwarzwald et al, 2009).