6 Mar 2019
Jacqui Matthews tackles a subject many equine vets do not find the easiest to raise with horse owners – even before anthelmintic resistance became an issue.

Image © Grigorita Ko / Adobe Stock
Even before anthelmintic resistance was added to the mix, intestinal worms were an issue in horses of all ages – and not always the easiest subject to cover with owners of yards or equines. Control plans and protocols are required, tailor-made for each yard, and based on up-to-date knowledge of parasite biology, local epidemiology and a sound history of a yard’s grazing management. They also need to be supported by diagnostic tests and a risk assessment of likely helminth transmission.
Intestinal worms are common and important pathogens of equids. Infections occur in animals of all ages, although youngsters and geriatric horses are more likely to exhibit clinical signs of worm-related disease.
Various worm species affect equids: foals are particularly susceptible to ascarid (Parascaris equorum) infections, and cyathostomins (small strongyles) are primary pathogens of adolescent and mature horses and ponies. Other parasites, the pinworm (Oxyuris equi) and the tapeworm (Anoplocephala perfoliata), can be problematical in some individuals. In all cases, disease is invariably seen in animals that have high burdens of worms.
Resistance to anthelmintics confounds effective control of equine helminths and is a particular issue in cyathostomins and P equorum. Cyathostomin resistance has been reported to all three broad-spectrum anthelmintic classes licensed to treat nematode infections in horses (benzimidazoles, tetrahydropyrimidines and macrocyclic lactones), with resistance to class one benzimidazole compounds almost ubiquitous in developed regions.
In some cyathostomin populations, multi-anthelmintic class resistance has been identified, with reduced effectiveness of macrocyclic lactones, pyrantel and fenbendazole, leaving no licensed options left for chemical control.
Macrocyclic lactone resistance is widespread in P equorum, with reports of resistance to the other two anthelmintic classes.
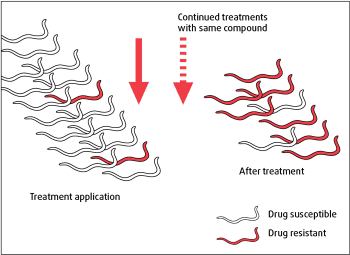
Resistance is inheritable, transferred from one worm generation to the next via the genome. In the presence of such resistance-conferring gene mutations, each time a dewormer is applied the majority of nematodes that survive treatment are resistant (Figure 1).
After repeated treatments with the same compound, resistant genotypes ultimately predominate and the anthelmintic fails to kill many worms, detected as low efficacy or the persistence of clinical signs in treated animals.
Movements of horses between sites facilitates the flow of anthelmintic resistance genes between populations, so appropriate quarantine treatment/management needs to be applied to stop this occurring.
Anthelmintic-resistant nematode populations do to not seem to revert to sensitivity, even when worms are not exposed to a specific drug class for many years. As yet, resistance has not been reported in tapeworm (A perfoliata), nor in the pathogenic large strongyle species, Strongylus vulgaris. That does not mean to say resistance in these species does not exist, just that it has not been detected in research studies or clinical reports.
The extent of benzimidazole, and now pyrantel, resistance reported in cyathostomins – combined with early indications of macrocyclic lactone resistance – is very concerning, given no prospect exists of new equine anthelmintics coming to the market.
For these reasons, cautious use of equine anthelmintics is now a priority and evidence-based control methods must be applied to reduce the frequency of treatments that act as a selection pressure for resistance.
To do this, control plans must be based on up-to-date knowledge of parasite biology, local epidemiology and a sound history of a yard’s grazing management, supported by results from diagnostic tests that provide information on which individual animals to target for treatment.
In all cases when giving advice, a risk assessment of likely helminth transmission should be performed. Every premise presents a unique situation, meaning control protocols should be tailor-made for each yard.
Cyathostomins are the most important equine metazoan parasites globally. The prevalence of cyathostomins approaches 95% globally, with most grazing equids exposed to these worms. Some individuals can harbour substantial burdens.
Fifty species have been described in the literature, but, in most animals, 5 to 10 common species make up the vast majority (more than 90%) of the overall burden.
The same common species are reported globally, with Cylicostephanus longibursatus, Cyathostomum catinatum and Cylicocyclus nassatus regularly reported as accounting for 70% to 80% of an individual’s total burden.
Horses are infected when they ingest third-stage larvae (L3) when grazing pasture. The L3 move into the wall of the large intestine and, once here, develop through encysted stages (early L3, late L3 and developing fourth-stage larvae; L4). The L4 emerge and moult to fifth-stage larvae, which mature to adult worms in the lumen of the caecum and colon.
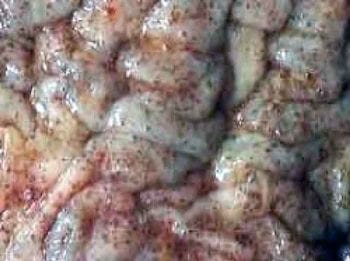
The encysted larval stages (Figure 2) are important in cyathostomin biology, as they can persist for prolonged periods (many months) and build up in massive numbers (several million), especially in the UK, in autumn and winter.
These larvae are not detected by commonly used faecal egg count (FEC) methods, so their presence goes unnoticed. Indeed, animals with sizeable encysted larval burdens often have negative or low FEC test results.
The larvae can emerge from the intestinal wall in large numbers to cause a severe colitis, larval cyathostominosis – the clinical signs of which range from mild weight loss and colic to a severe protein-losing enteropathy with diarrhoea and death in up to 50% of severe cases. This syndrome is not associated with a particular cyathostomin species.
By the time most horses reach adulthood, they develop immunity to cyathostomins and control their burden well; this is reflected in the number of eggs shed in dung.
The usual pattern in a group is that 80% of individuals have low burdens and low worm egg shedding. The remaining 20% have higher burdens and usually shed around 80% of the eggs detected at a given sampling (Figure 3).
The proportions of egg shedding dynamics vary depending on management, with more horses shedding higher levels of eggs when kept under management conditions that promote high larval challenge on pasture (for example, in groups grazed at a high stocking density on permanent pasture where no dung removal takes place).
The dynamics of worm egg shedding in equine populations mean it is usually a small number of animals that contribute the majority of eggs on to pasture at any given time. This can be exploited to target anthelmintics to reduce treatment frequency (and hence selection pressure for resistance) in a given population.
Each animal’s susceptibility to worms is likely to be maintained over time; research studies demonstrate reasonable temporal consistency in egg shedding in individuals.
Strongyle infection levels are usually higher in younger horses where immunity has not developed. Such populations have higher levels of strongyle egg shedding overall and pastures grazed by these groups will be more contaminated with worms; this needs to be taken into account when designing control programmes.
Large strongyle adult worms are found in the large intestine, but unlike cyathostomins, the larval stages migrate within the host. The most important large strongyle is S vulgaris – the larval stages of which develop in the cranial mesenteric arteries. Here, these worms can lead to blocked arteries, resulting in intestinal infarction and severe colic.
Large strongyle adult and larval worms are very sensitive to macrocyclic lactones (ivermectin and moxidectin) and, because of high usage of these types of dewormers in the UK, clinical disease due to S vulgaris is rarely reported.
Studies in Denmark have indicated increased detection of S vulgaris, which has been associated with the substantial reduction in macrocyclic lactone applications following a change in legislation whereby horses are only treated based on the results of diagnostic tests.
P equorum is an important worm globally. Significant burdens are only observed in animals younger than 18 months, as most horses develop strong immunity to the parasite. The large (up to 10cm) adult worms live in the small intestine and females release substantial numbers of environmentally resistant eggs in their lifetime.
Infective larvae develop within eggs, which are then ingested when foals graze. The larvae hatch out in the small intestine, then migrate via the liver and lungs to be coughed up and return to the intestine. Eggs are excreted around 10 weeks after initial infection.
These round, thick-shelled eggs can be detected using standard FEC flotation tests. P equorum is common on breeding establishments and is a particular problem in foals grazed on permanent pastures.
Clinical signs of infection include respiratory disease, poor condition and colic when infection intensity is high. P equorum-induced colic has a poor prognosis. As already indicated, anthelmintic resistance is an issue in this species – particularly resistance to the macrocyclic lactone compounds, ivermectin and moxidectin.
Another nematode commonly reported in the UK is O equi, the pinworm. Indeed, signs due to pinworm in individuals are increasingly reported. The reasons for this are not known, but could be due to anthelmintic resistance, poor efficacy of the compound selected for treatment or increases in environmental conditions that enable the eggs to survive better outside the host.
Resistant pinworm eggs are ingested from gates, fences, stable doors and troughs, with most intra-host stages found in the terminal large intestine. Female adults migrate to the perineal area to lay eggs, which are transferred to the environment when the horse rubs. Most horses and ponies show no evidence of pinworm infection, but in some individuals (for reasons unknown), persistent infections result in clinical signs – mostly pruritus, leading to, sometimes severe, damage in the perineal region and tail head.
Anthelmintics with licensed efficacy against larval and adult pinworm are ivermectin, moxidectin and fenbendazole. Pyrantel embonate has a licence against adult stages only. Anecdotal reports have suggested a “lack of effectiveness” for all anthelmintic classes available.
Exemplary hygiene is required to reduce egg infection in the environment. Thorough cleaning with a horse-friendly disinfectant, after removal of all bedding, will reduce infection risk. Anywhere animals rub against, and potentially dislodge, pinworm eggs requires cleaning, including stable doors, walls, mangers, troughs, gates and fences.
Thorough daily cleaning of the perineum and tail-head of individuals must be undertaken, together with tail plaiting. The application of Vaseline to the area may help dislodge female worms and reduce the number of eggs sticking to the coat. Pre and post-treatment monitoring for infection by “tape testing” is important in assessing effectiveness of the treatment and control measures applied.
A perfoliata has a high population-level prevalence in the UK, but most horses carry a negligible or low burden. Similar to nematode infections, some individuals are predisposed to higher burdens, which can lead to clinical disease, primarily, and both spasmodic and impaction colic.
This parasite has an indirect life cycle that involves development of tapeworm larval stages in Oribatida mites. Horses ingest infected mites containing tapeworm cysts. The intra-host stages develop and mature at the ileo-caeco-colic junction.
Routine FEC flotation methods are not particularly sensitive for tapeworm eggs and shedding of eggs is variable over time. In the UK, two diagnostic tests (a blood test and a saliva test) are available that rely on measurement of specific antibody levels to tapeworm antigens.
The liver fluke (Fasciola hepatica) is another helminth that can infect horses and ponies. Sheep and cattle are likely sources of infection of the parasite, which, as with tapeworm, has an indirect life cycle – in this case involving a mud snail intermediate host.
Liver fluke is recognised as an increasing problem in ruminants due to an increase in prevalence and the spread of resistance to the most potent flukicide, triclabendazole.
With increased reliance on co-grazing horses with ruminants, owners should be made aware of the potential threat liver fluke poses. The mud snail intermediate hosts persist for extended periods on wet paddocks; horses become infected with the tough metacercariae that develop after shedding of larval stages from infected mud snails. The metacercariae hatch in the small intestine and larvae migrate through the liver to develop to adult worms in the bile ducts.
Clinical signs are not usually seen in infected equids, but some horses present with raised serum liver enzymes with weight loss and malaise. In cases where infection is identified, horses need to be treated by a veterinary surgeon under the cascade2, as no equine flukicides are licensed in the UK.
Because of its higher safety margin and wide spectrum of activity against different fluke stages, triclabendazole is recommended. However, with increasing reports of resistance to triclabendazole in F hepatica in UK sheep flocks, the treatment may not work. For this reason, monitoring for infection by FEC sedimentation testing three weeks after treatment is recommended, as well as observation for a reduction in clinical signs and serum liver enzyme levels.
Where triclabendazole is found to be not effective, treatment with closantel may be considered. This flukicide is not effective against larval stages, so a follow-up treatment is required 8 to 10 weeks after the first to kill adult fluke that develop from larvae that persist beyond the first treatment. Be aware of overdosing with this compound; signs of toxicity include blindness, anorexia and ataxia.
Control must tackle transmission via mud snails and should focus on removing access of horses and ponies to potential mud snail habitats.
Dictyocaulus arnfieldi (the equine lungworm) and Strongyloides westeri (a small intestinal nematode of young foals) are found in the UK; clinical disease due to these is relatively rare compared to the aforementioned species and is only observed in certain situations (for example, when horses co-graze with donkeys – lungworm – and when foals are stabled under unhygienic conditions, S westeri).
Managing pasture nematode challenge is key to sustainable control. Plans for improved pasture management need to take account of yard management, local climatic conditions and worm life cycles.
Fields should not be overstocked. The recommendation is one horse per one to two acres (0.4 to 0.8 hectares). When resting pastures, it is important to allow an adequate time for significant mortality of free-living worm stages. In the UK, resting for six to nine months (over winter until the middle of the next summer) is recommended before pastures can be assumed “safe”.
Dung should be removed from pasture, particularly when paddocks are grazed intensively. Dung removal twice per week has been shown to significantly decrease the number of strongyle eggs shed by resident equids compared to animals on paddocks where no dung removal was practised. In these studies, on dung-removed paddocks, the frequency of targeted anthelmintic treatments was much lower, as egg-shedding levels across the population were reduced3.
It was subsequently shown equids on yards with no dung removal had shorter strongyle egg reappearance periods after moxidectin treatment than individuals resident on yards where dung removal is practised4. Therefore, on yards where there was no dung removal, horses received more frequent treatments, therefore increasing selection pressure for resistance.
Dung should be properly composted if it is to be subsequently spread on to grazing land. It is suggested it should be composted for at least six weeks, with regular turning so a minimum temperature of 50°C to 70°C is reached that will kill helminth eggs.
Co-grazing with ruminants can be undertaken; most helminths are species-specific, but keep in mind that, where there are mud snail habitats, horses can become infected by liver fluke on land contaminated by infected ruminants. In these circumstances, improved drainage and flukicide treatment/efficacy monitoring in livestock are essential.
Harrowing is not recommended in the UK; in the main, the climate is neither sufficiently dry nor hot enough to kill nematode larvae spread across paddocks. The practice is more likely to result in the spread of live larvae, as well as P equorum eggs.
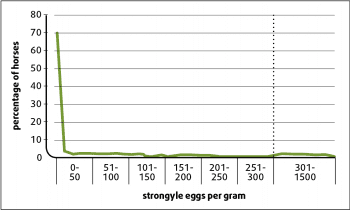
Most adult horses have low worm burdens and low egg shedding. This distribution facilitates protocols that reduce anthelmintic usage by using FEC tests to inform on treatment requirements of individuals from spring to autumn.
Targeted treatment protocols must incorporate good grazing management. As already indicated, cyathostomin-encysted larvae do not produce eggs, so are not detectable by FEC tests.
For accurate FEC testing, samples should be collected from freshly passed dung (namely, dung passed within the previous 12 hours).
A representative sample must be collected, as nematode eggs are not evenly distributed across a dung pile. Therefore, it is recommended that 30g to 40g (a good handful) be sampled by taking small quantities of dung from three different balls across the pile.
The sample should be mixed well before placing in a labelled zip-lock bag that has all air expelled before sealing. If samples are not taken to the laboratory immediately, they should be refrigerated (4°C) until transfer. Samples must be processed within five days of collection.
At the laboratory, a method with a low egg detection limit should be used. Guidelines have been published on how to increase the sensitivity of a standard modified McMaster flotation test from 50 to 15 eggs per gram (EPG)5. The sample must be well mixed prior to sub-sampling for the FEC method.
The anthelmintic compound used for the previous treatment determines when an FEC test should be performed; tests should be done just after the strongyle egg reappearance period for the last compound used. Strongyle egg reappearance periods for anti-nematode anthelmintics are:
If positive FEC tests are observed in the majority of a population before or around the egg reappearance period, resistance should be suspected and an FEC reduction test (FECRT) should be undertaken to assess efficacy. Strongyle eggs reappear more quickly after treatment in young horses; this should be taken into account when selecting FEC testing frequency in such groups.
Anthelmintic effectiveness must be tested as part of a sustainable control programme. This can be undertaken by performing an FECRT. To do this, FEC tests need to be performed on all horses at the time of treatment. Tests should be done following the aforementioned guidelines.
The more horses included in an FECRT, the more robust the data obtained. Ideally, 10 or more horses should be tested, but it is accepted this cannot always be achieved in practice. It is important to use as sensitive an FEC test as possible.
Dung samples should be collected from all horses 14 to 17 days after treatment, and handled and processed in the same manner as the day of treatment samples. Try to ensure samples are obtained at the same time of day. Once the second FEC is completed, efficacy can be calculated using this equation:
Percentage of FEC reduction = ([mean day 0 FEC – mean day 14-17 FEC] / mean day 0 FEC) × 100
Guidelines suggest that, for fenbendazole and pyrantel embonate, a mean FEC reduction of greater than or equal to 90% indicates efficacy. For ivermectin and moxidectin, a mean FEC reduction of greater than or equal to 95% signifies efficacy.
If the mean group reduction is lower than these thresholds, the data should be scrutinised to identify if one or two individuals contribute to the higher post-treatment results. In such cases, the animals may have been misdosed and retesting of these horses is recommended.
If poor efficacy is detected, it should be reported to the VMD’s Suspected Adverse Reaction Surveillance Scheme6.
As most adult horses have no or negligible burdens of A perfoliata, anti-cestode treatments should be applied on the basis of a diagnostic test result.
As both tests rely on detection of tapeworm-specific antibody, a sufficient amount of time needs to have lapsed from the previous treatment to enable historic antibody levels to reduce before the next test is applied. Details of timing for the tests are available on the respective websites.
Liver fluke eggs are shed in faeces, but these heavy eggs cannot be detected using standard FEC flotation methods.
Diagnosis of patent infections can be made using faecal sedimentation tests, but these are not particularly sensitive in equine infections and several samples may need to be analysed before a positive test is obtained.
A serum test for equine fasciolosis is now available from the University of Liverpool9.
Equine dewormers should be applied as:
For all treatments, best practice must be applied; the dose administered must be based on an accurate assessment of weight (using weigh scales or a good quality girth tape) and anthelmintics must be stored in accordance with their summary of product characteristics10.
In the UK, targeted treatments should be applied between spring and autumn. Here, FEC tests are undertaken to identify which horses need treatment on the basis of strongyle egg shedding level.
Regular testing identifies individuals most susceptible to infection, and hence at risk of disease. These tests do not provide any estimation of total worm burden within individuals.
No published guidelines define the cut-off FEC value to base anthelmintic treatment on, but most sources recommend treatment of horses shedding between 200 and 500 EPG.
The threshold selected should depend on a history of grazing management; for example, a higher FEC threshold (500 EPG) could be used in adult horses on clean pasture at low stocking density compared to a group of yearlings grazed at high stocking density on paddocks with no dung removal (200 EPG).
Vets (and, indeed, all prescribers) should use their knowledge of the farm’s management and anthelmintic treatment history to make a sound judgement on what EPG threshold to select for treatment.
For the FEC-directed treatment of strongyle infections, ivermectin or pyrantel can be used from spring to autumn. It is recommended efficacy be assessed once a year using a FECRT. This is particularly important when prescribing pyrantel due to the level of anthelmintic resistance to this compound already identified in cyathostomin populations in the UK.
The aforementioned tapeworm tests can be used to inform application of anthelmintics to target A perfoliata. In the UK, anthelmintics with licensed efficacy are praziquantel and pyrantel embonate (at twice the dose used for nematode treatments). Ideally, anthelmintics with a narrow spectrum should be used for tapeworm treatment unless treating concurrently for nematode infection based on time of year or following results of a nematode FEC test.
Praziquantel-only products for equines can now only be prescribed by veterinary surgeons. By using this diagnostic-led approach, anthelmintic resistance selection pressure will be reduced, as substantially less treatments will be applied in a population overall.
In the UK, it is recommended strategic treatments be applied in late autumn/early winter to target cyathostomin-encysted larvae and large strongyle larvae. These stages of worms are not detectable using existing tests.
Two anthelmintics have licensed efficacy against all stages of cyathostomin-encysted larvae; moxidectin and fenbendazole (when administered in five consecutive daily doses). Levels of cyathostomin resistance to fenbendazole are such that use of the anthelmintic for this purpose is not recommended unless a population has been shown to be sensitive to the compound by efficacy test. Therefore, moxidectin is recommended for larvicidal treatment.
In some populations – for example, in groups of yearlings on pasture over a mild winter – a second moxidectin treatment in late winter/early spring may be required to target developing larvae from infections ingested over the winter.
The decision to treat should be based on knowledge of the group’s grazing conditions supported by FEC testing 12 to 14 weeks after the application of the previous treatment. An antibody-based test is being developed for detecting intra-host stages of cyathostomins, which will help inform the application of “larvicidal” treatments in future.
To mitigate spread of anthelmintic-resistant worms from one yard to another, all incoming equids should be stabled and treated with a moxidectin/praziquantel combination, which will target as many helminth species and stages as possible.
Horses should be stabled for three days after treatment. Ideally, an FEC test should be undertaken 14 days later to assess if the compound has been effective in reducing nematode egg shedding above 95%. Thereafter, the animal can join the ongoing worm control plan.
The increasing prevalence of anthelmintic resistance is a real challenge to veterinarians in the control of equine parasites.
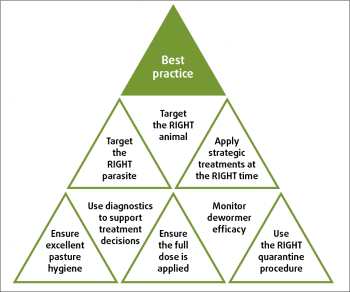
The application of evidence-based control methods (summarised in Figure 4) – based on a sound knowledge of the biology and epidemiology of infections, combined with diagnostic tests – will help slow resistance development by substantially decreasing the number of anthelmintic treatments applied over time.
Veterinarians need to fully engage in the implementation of these methods of worm control to try to preserve efficacy of the effective anthelmintics.