31 Jul 2017
Tony Andrews discusses methods of defining and treating this condition by reporting on a selection of available studies.

Image: © Fotolia/vsarts.
The latest edition of Veterinary Medicine was published this year (Constable et al, 2017a; 2017b), and includes summer mastitis information in the Trueperella pyogenes and head fly Hydrotaea irritans sections.
However, the most recent edition of Bovine Medicine is a disappointment on this topic in that while it has a very useful chapter by Biggs (2015) concerning a farm audit for udder health, mastitis and milk quality, no section on summer mastitis, T pyogenes or head fly involvement appeared to feature. This deficit is rectified in Andrew Biggs’ own book, Mastitis in Cattle (2009), where a whole chapter is devoted to the topic, and summer mastitis receives a full and helpful chapter in Mastitis Control in Dairy Cows (Blowey and Edmondson, 2010).
Thus, it appears the subject is not forgotten and is still of some importance, especially as it does not really lend itself to the categorisations usually used to define mastitis or its control.
As it appears little information is new, it does suggest little research interest exists in the topic, possibly because most cases are relatively easy to detect, appear to be seen only in certain countries and the likely outcome is known. However, many intriguing questions remain that really do appear to need answering or information reaffirmed.
The term “summer mastitis” is often relatively loosely used as, particularly in other countries, it often embraces all cases of T pyogenes infection of the udder.
Hillerton (1992) defined the problem as: “Summer mastitis is an acute or peracute multifactorial infection of the non-lactating bovine mammary gland, although clinicalsigns are often not obvious until parturition.”
Another useful working definition, at least for the British Isles, is of it being a severe clinical mastitis occurring in non-lactating cattle usually at pasture during the summer months (Berry, 1998). Besides both beef and dairy non-lactating cows being affected, cases occur in pregnant and non-pregnant heifers, calves and bulls.
Most reports concerning summer mastitis appear to have been made in the Northern Hemisphere in countries such as the British Isles, Europe and the US. However, Ruegg et al (2014) indicate the problem is only one of Europe and Japan, and most cases elsewhere do no fit the aforementioned case definition. Reports are rare in the Southern Hemisphere and, although in Australasia summer mastitis is considered to be seen sporadically, most cases appear to be of T pyogenes and are seen in lactating cows with damaged teats (Malmo et al, 2010).
The incidence annually in the 1980s was put at between 20% and 60% of dairy herds in many northern European countries (Hillerton, 1987). Reports for the six-year period between 1978 to 1983 showed, on average, about 47% of English and Welsh dairy herds were affected, with three cattle infected in each herd (overall incidence of 0.9%), with 0.7% of cows and 1.5% of heifers involved (O’Rourke et al, 1984). More cases occurred in the west (north-west, Wales, west midlands) than the east (East Anglia, south-east). The same authors looked at dairy herds in 1983 and found an incidence of 1.2% in cows, 1.9% in pregnant heifers and 0.6% in non-pregnant heifers.
In Ireland, the prevalence of infection in cows ranged from 4.5% to 10% in dairy herds in the Cork and Dublin areas (Egan, 1986). However, a more detailed study of 373 herds showed that, over a six-year period, summer mastitis occurred in 63.8% of all herds surveyed. In herds with appropriate cattle, 53.4% of herds having at least one dry cow affected, 29% at least one pregnant heifer and 8.9% a non-pregnant heifer; and an average annual herd incidence of 7.4%, 6.3% and 1.1%, respectively (Egan, 1994). As the studies were more or less contemporary, it might suggest summer mastitis was more of a problem in Ireland than in England and Wales.
The disease is often called the “August bag”. A study in Yorkshire that showed dry cow intramammary infusions every three weeks was highly efficacious in preventing the disease also indicated most summer mastitis cases occurred in the first few weeks of August (73% in the month overall), with 15% in July and 12% in September (Edmonds and Welsh, 1979). August also produced the highest number of cases in Ireland (58.7%), followed by July (31%) and September (9.9%; Egan, 1994).
Certain farms and particular fields are often involved in the disease. The areas tend to be low lying, sheltered, containing trees and are close to a stream or other water course, with well-drained soil. In some cases they occur on land with weeds, thistles or potentially abrasive plants that have, at times, been suggested to provide initial teat damage for attraction of flies and infection to occur.
Studies on disposals from dairy herds showed mastitis formed a major cause of disposals, but the disease was not separated into its various causes (Whitaker et al, 2000; 2004). At the time of the second paper, mean annual mastitis rates were 38.2% and these were slightly lower than in another study made at about the same time in a smaller number of herds (Esslemont and Kossaibati, 2002).
The author has not found any recent surveys on summer mastitis that span a number of years and, as dairy cow management has altered greatly since the 1990s, it is wondered whether this has also affected the disease. It is probable it has and the author’s impression is the disease is less commonly seen than in the past, although no proof of this exists. It may be surveys have been undertaken to show the present level of summer mastitis, but the author has not discovered them to prove or refute this point.
Previous history:
Present situation:
On the basis of previous/present history, can vulnerable animals be kept away from areas of previous/current problems?
Check fields used:
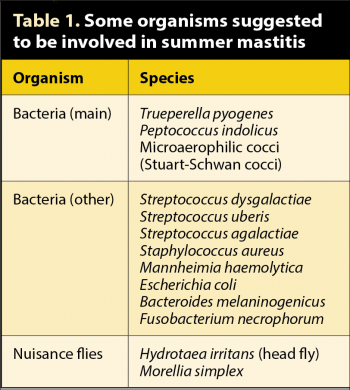
The disease is complex in its aetiology and debate exists about the micro-organisms involved, as well as transmission. Table 1 indicates some of the suggested contributors to the disease. Discussion still occurs about the causes of summer mastitis. Most consider the main bacterium involved to be T pyogenes, which has had a number of name changes over the past few years, starting with Corynebacterium pyogenes then Actinomyces pyogenes and Arcanobacterium pyogenes. An anaerobic bacterium, Peptococcus indolicus, as well as other microaerophilic cocci, are isolated from some summer mastitis cases, usually in association with T pyogenes. They do appear to be involved, but are often not detected because samples are not cultured in lowered oxygen or anaerobic conditions.
In several studies, many samples have shown mixed bacterial infections; in some, no pathogen can be isolated, for example Yeoman and Warren (1984). Experimental infection of cows with T pyogenes and P indolicus in mid-lactation was usually unsuccessful, but their establishment increased seven days before drying off and it occurred in all quarters infected at drying off (Hillerton and Bramley, 1989). As T pyogenes is commonly found in abscesses in cattle, it is considered infection could be either externally via the teat orifice or internally via haematogenous spread from other parts of the animal (Biggs, 2009).
As the disease tends to be seen in the summer and in reasonably specific areas, it indicates potential arthropod or other vector involvement in the disease. It is generally accepted the sheep head fly, Hydrotaea irritans, is involved. This fly is mainly found in Britain and Europe, and similar to the horsefly in size, but with an olive green body. It tends to be active in the summer months, and frequents the belly and udder of cattle (Hillerton et al, 1984). The fly avoids low relative humidity and strong winds (Tarry and Kirkwood, 1974).
H irritans is a non-biting or nuisance fly, and evidence of how it transmits the problem has, at times, been equivocal. Its life cycle was mainly determined by Tarry and Kirkwood (1974), who showed male flies predominated in June and July. In early August a massive swing is made to females, which are avid protein seekers to allow egg production. Larval development occurs in the soil and only one generation of flies appears to be produced in each year.
In south-west Scotland, another nuisance fly, Morellia simplex (13.9%), was considered to be second in importance to H irritans (69%) in cattle at pasture (Titchener et al, 1981). The prevalence of biting flies was low, with the most common being Haematobosca stimulans (4.5%), with only minimal numbers of others, such as clegs (Haematopota pluvialis) and horse flies (Hybomitra distinguenda). Strong circumstantial evidence of H irritans involvement was shown on six southern England dairy farms in two successive years (Bramley et al, 1985).
Successful transmission of T pyogenes, Streptococcus dysgalactiae and Streptococcus agalactiae infection to H irritans was reported to have occurred in 1977 (Wright and Titchener, 1977). They also suggested the mustelid fly was not just a vector, but became infected with T pyogenes and S dysgalactiae. Transmission of summer mastitis to dry cows was also shown experimentally using H irritans flies that had previously fed on the teats of naturally occurring cases (Tarry et al, 1978). However, others (Hillerton et al, 1990; Madsen et al, 1991) found it hard to produce proof H irritans was the main vector of the disease.
Others were able to confirm the earlier work by producing summer mastitis in heifers, some of which had their teats damaged (cut) prior to fly exposure (Chirico et al, 1997). On farm it seems once one case of the disease occurs others may follow because of the attraction of the affected quarter to flies.
The problem is usually easy to diagnose, affecting non-lactating cattle and younger animals in the summer months. The first signs are usually a collection of flies attracted to the animal’s teat and this is often missed. The teat will initially swell and increase in length, which is particularly noticeable in heifers. Often, this can be several days before the udder swells and the animal becomes ill. The affected quarter becomes hard, swollen and painful, and the secretion expressed is thick yellow and smelly.
The animal often tends to separate from the others, it will kick at the affected quarter, and signs of slight lameness may initially occur and become more pronounced. It is not long in this process before varying degrees of systemic signs present due to toxaemia, including pyrexia, anorexia and loss of condition. Occasionally, affected cattle will abort and, very occasionally, cattle die or are euthanised.
By the time the infection is noticed the quarter will be irreparably damaged and a write-off. This means the animal will probably not be suitable for milking, as three-quartered cows tend to cause practical problems in the parlour.
The only case the author has read where the cow with a severe summer mastitis became better is in one of James Herriot’s tales, where the farmer was desperate to save the quarter and milked her throughout the night. Occasionally, the quarter recovers, but usually only in mild cases detected early on.
In essence, treatment is to save the life of the cow, but not the quarter.
The animal should be separated from the herd and kept indoors until recovered so the infection does not spread. Madsen et al (1991) showed clinical cases of summer mastitis were sources of bacteria to infect other cattle for more than three weeks, despite treatment and teat amputation.
As the animal will have a reduced appetite or anorexia, it should be tempted with highly palatable feeds and the painful udder means it should be bedded on deep straw to allow the animal some comfort when lying down. Usually parenteral antibiotics work well in this problem. T pyogenes does not generally have a major antimicrobial resistance problem, so most of the common antibiotics are usually effective.
The animal will be in considerable pain from the swollen gland and also toxic, so NSAIDs should be used.
However, the quarter will be non-functional and can often be regarded as a large abscess. If the udder can be bathed in warm water and the material milked out of the teat as often as possible, it will reduce the toxins entering the animal’s body.
In some cases, it is necessary to treat the swelling by lancing it in the same way as an abscess, making a sufficiently large wound to allow regular flushing out of the pus, ideally with warm water. The wound should not be allowed to close up until the gland is starting to heal and no further pus is being produced.
As the quarter is inevitably lost and if the infected secretions cannot be removed through the teat, the drastic measure of removing the teat can be helpful. However, when this is done, it must be remembered a rim of teat tissue is left in case it haemorrhages.
When this occurs it can be very difficult to arrest the bleeding unless a teat rim is available to work on. Usually, an effective method to allow drainage is to cut the teat longitudinally (Blowey and Edmondson, 2010).
All vulnerable animals in suspect fields should be thoroughly checked at least twice daily.
Cows – methods can include:
Heifers – methods can include:
Cows/heifers fly control:
Change dry period (usually not economically practical).
It is best to determine the risk and, if there is one, where possible undertake avoidance strategies. However, many other factors also need to be considered in the management of the dry period to prevent metabolic problems and clinical mastitis in cows after calving.
In the latter case, the reduction of clinical mastitis – by adopting a pasture grazing rotation policy involving the use of a paddock for a maximum of two weeks before allowing the area to be non-grazed for a period of four weeks (Green et al, 2007) – could mean fields potentially capable of harbouring summer mastitis vectors are incorporated into the grazing plan. Care needs to be exercised as to when such areas are used.
Some of the factors to be considered are presented in Panel 1.
In many instances, it should be possible to lower or remove the risk of problems. However, on many farms, it is heifers and younger stock, or dry cows, that are kept in the fields furthest from the farm buildings, as they are too far for the milking cows to graze and commute for milking. Often, the fields used are unsuitable for grass conservation (silage or hay) because of their size, access or topography, so their use by vulnerable cattle may be necessitated. Although sheep could use these pastures, they will be very susceptible to head fly and other nuisance fly damage, especially if they are horned. Such affected sheep can suffer greatly from the problem, which can be a severe welfare issue.
In theory, it is suggested fly control at pastures should be based on a system of integrated parasite control (Dent, 2000), which, in practice, is often very difficult to achieve.
Thus, in this situation, it is a question of conflicting priorities and, in the end, a balance between the animal welfare of the animals (cattle or sheep), possible human health problems and potential environmental consequences from some ectoparasitic treatments, as well as maintaining the profitability of the farm (Milne et al, 2008).
If it is essential for vulnerable cattle to use suspect fields during the fly season then, possibly, some of the suggestions in Panel 2 could be considered.
Dry cow therapy has been partly instrumental in reducing mastitis levels in dairy cows at the end of lactation from about 60% to between 10% to 20%, depending on the farm and country (Hillerton, 2017). Much time and thought is concentrated on reducing levels of overall animal antibiotic usage in an attempt to slow down, or reduce, antimicrobial resistance development.
In consequence, the blanket use of antibiotics in animals as a preventive strategy has been questioned, including dry cow therapy. This has resulted in the recommendation such therapy should be targeted at those dairy cows that require it and should not be used on those animals where it is probably not required. Alterations in terminology for dry cow terminology have been suggested (Hillerton et al, 2017). However, decisions on whether to prescribe blanket dry cow therapy or selective dry cow therapy are complex for vets (Higgins et al, 2016) and more difficult for farmers.
As this article previously stated on controlling summer mastitis, strategies should be used to avoid placing vulnerable animals in fields where nuisance flies and, especially, H irritans might be present. However, management theory and what is practical cannot always coincide and, in such circumstances, it is suggested dry cow treatments can probably be justified because of the severe welfare and economic effects of the disease. While this does mean the use of blanket dry cow therapy for all those cattle in a vulnerable environment, it will only involve animals over a period of a few months and not the entire year.
Thus, this does appear to fall within the apparent prophylactic use of antimicrobials guidelines, such as those of the BCVA (2016), which states: “The BCVA is recommending that prophylactic use of antibiotics is to be avoided wherever possible without compromising animal welfare. Where it is used it should be regarded as an interim measure, while alternative management and/or vaccination strategies are implemented.”
However, at present, until more effective fly control measures are in place, the second sentence may be difficult to achieve. In addition, when antimicrobial therapy is being used for summer mastitis control then it should form part of the farm’s comprehensive herd health plan.
This article shows, although summer mastitis still exists and can be a considerable problem on some farms, little new information on the problem is available.
The author wishes to thank the RVC library staff, especially Sally Burton and Louise Avie, for finding some of the older references, and Andy Biggs, Andrew Bradley and Alastair Macrae for providing helpful information.