13 Aug 2024
Anna Bruguera reviews the various clinical presentations of BVD and highlights the importance of applying control measures for improving animal health, welfare and herd productivity.

Bovine viral diarrhoea virus (BVDV) significantly impacts cattle health, welfare and production due to its wide range of clinical presentations. The severity of these effects varies depending on the strain’s virulence, infection timing and the cattle’s immunity. BVDV spreads through direct and indirect contact, with vertical transmission from dam to fetus resulting in persistently infected (PI) cattle, which are the main reservoirs of the virus. Although most BVDV infections are subclinical, the virus’ immunosuppressive effects and reproductive losses are particularly consequential.
Effective BVDV control starts with identifying and removing PI cattle, followed by regular herd monitoring and strict biosecurity protocols. Vaccines against BVDV have been shown to be effective and useful to reduce the impact the virus has on herd production, but should be used as part of a comprehensive strategy targeting PI eradication and prevention.
BVDV has been eradicated in several European countries. In the UK, BVDV infections remain common in regions without – or that have just started – compulsory eradication schemes (England and Wales), while the virus’ prevalence has significantly decreased in areas with long-standing mandatory programmes (Northern Ireland and Scotland). In both situations, vets play a crucial role in encouraging farmers to adopt BVDV control measures to eliminate the virus and minimise losses in endemically infected herds, and prevent potential outbreaks in naive herds.
Bovine viral diarrhoea (BVD) is a disease of cattle that can manifest in a wide variety of clinical presentations depending on the production stage and immunity of the affected animals.
Consequently, BVD virus (BVDV) has a significant negative impact on animal health and welfare, and causes important economic losses to the cattle industry (Richter et al, 2017; Yarnall and Thrusfield, 2017). While BVDV has been successfully eradicated from multiple European countries (Moenning and Becher, 2018), each UK nation is at a different stage in the BVDV eradication journey (Bruguera Sala, 2024).
In nations with long-standing compulsory eradication schemes, such as Scotland and Northern Ireland, the disease prevalence is decreasing and clinical cases of BVDV are becoming less frequent. On the other hand, in England, where BVDV monitoring and control remains voluntary (UK Government, 2024) and Wales, which has just moved to compulsory BVDV testing (as of July 2024; Welsh Government, 2024), BVDV infections remain common and are likely still causing significant losses to infected herds (Animal Health and Welfare Wales, 2023; Prosser et al, 2022).

In both scenarios, it is crucial for veterinarians to stay vigilant in identifying the clinical presentation associated with BVD. This vigilance is essential for diagnosing potential new outbreaks in previously negative herds and raising awareness of the ongoing losses BVD can cause in endemically infected herds. Veterinarians play a key role in encouraging farmers to take proactive measures to control BVDV.
This article reviews the various clinical presentations of BVD and its associated costs, at the herd level, aiming to highlight the importance of applying control measures for improving animal health, welfare and herd productivity.
To understand the impact BVD has on cattle health and welfare, it is essential to understand how the virus operates. BVDV is a member of the Flaviviridae family, genus Pestivirus.
The two species of BVDV are BVDV-1 and BVDV-2 – each with several identified strains that exhibit varying degrees of virulence. Both species present in two biotypes: cytopathic (CP) and non-cytopathic (NCP), although these are not linked to the virulence level.
The virus is primarily transmitted through direct contact between infected and naive animals, and by vertical transmission from an infected dam to its fetus, as well as through contaminated semen or embryo transfer. Naso-oral and vertical transmission routes are the most common and significant for disease spread. Indirect contact via fomites also plays a role in transmission. Viraemic animals excrete the virus in most body fluids, including blood, nasal and ocular secretions, milk, urine, faeces, semen, and fetal fluids and membranes.
When immunocompetent naive cattle are exposed to BVDV, they develop an acute infection and transient viraemia, which can last between 3 and 12 days (Pedrera et al, 2012). Most acute BVDV infections remain subclinical, but clinical signs can vary from mild to severe, depending on the virulence of the infecting strain (Lanyon et al, 2014).
Clinical signs of acute BVDV infections include pyrexia, depression, anorexia, diarrhoea and respiratory signs (Lanyon et al, 2014). Haemorrhagic syndrome is a severe presentation of acute BVDV infections, associated with highly virulent strains, which also causes thrombocytopenia and mucosal ulceration (Liebler-Tenorio, 2003).
Two to three weeks post-infection, transiently infected (TI) animals develop BVDV antibodies and become immune to virus. Immunity after natural exposure to BVDV could last for years (Fredriksen et al, 1999; Müller-Doblies et al, 2004).
BVDV primarily infects white blood cells, and causes atrophy of lymph nodes, Peyer’s patches, enterocytes, the thymus and tonsils (Chase, 2013; Pedrera et al, 2012). This tropism for lymphoid tissues results in immunosuppression, making affected animals more susceptible to secondary diseases.
The BVDV replication in lymphoid tissues is particularly marked in the lung and mesenteric gut, which is why pneumonia and diarrhoea are common complications associated with BVD (Bolin, 2002; Hay et al, 2016).
The virus has also been linked to an increased incidence of mastitis (Berends et al, 2008; Yue et al, 2021a and 2021b) and, as it spreads to all body tissues, could also elevate the prevalence of other disorders. As most primary acute BVDV infections remain subclinical, the immunosuppressive effects and the impact on reproductive function are the most significant consequences of BVDV.
If the exposed naive animal is a breeding or pregnant heifer or cow, BVDV can affect all stages of the reproductive cycle: from causing infertility, early embryonic deaths and abortions, to the birth of deformed and/or stillbirth calves. The reproductive consequences of BVDV infections are summarised in Figure 1.
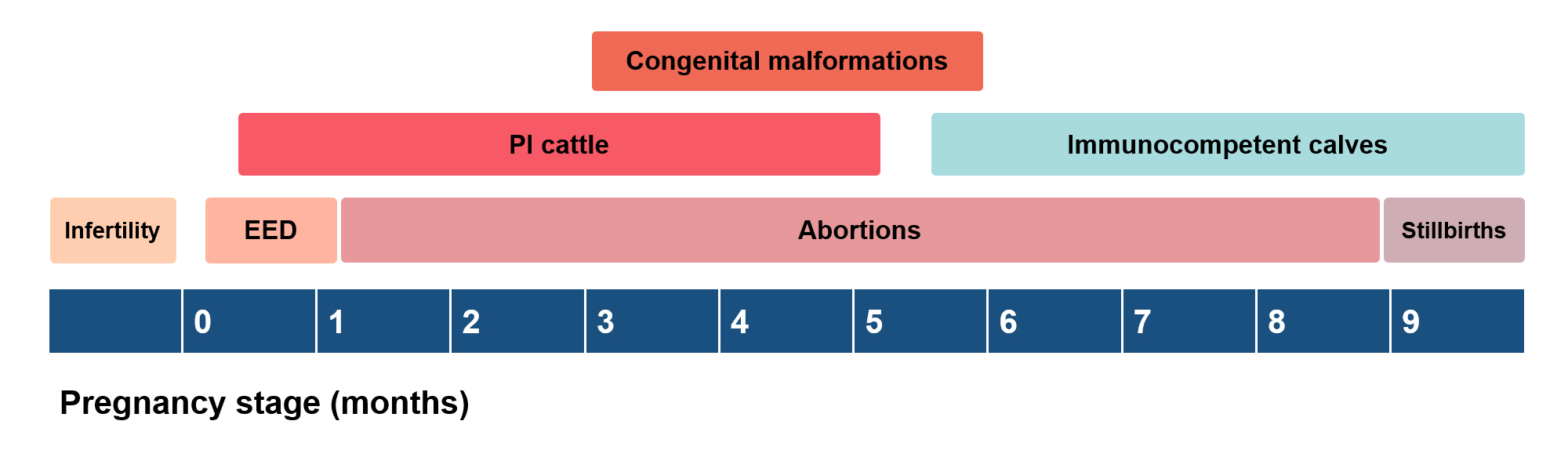
Cerebellar hypoplasia is the most frequently reported congenital malformation associated with BVDV, but the virus can also cause hydrocephalus, ocular degeneration, microphthalmia, congenital cataracts, brachygnathism, thymus atrophy, renal dysplasia, and bone and lung growth retardation (Lanyon et al, 2014).
If a naive pregnant dam is infected before the fetus’ immune system is functional, vertical BVDV transmission will result in the birth of a persistently infected (PI) calf. Persistent infections are caused solely by NCP BVDV strains and can occur between 18 and 125 gestational days, as bovine fetuses become immunocompetent by day 150 (Grooms, 2004).
PI cattle are immunotolerant to BVDV and serve as the primary source of new infections, constantly shedding the virus in most body secretions (Houe, 1995). PI cattle can also develop BVDV antibodies if exposed to a strain different to the one that caused the persistent infection, but they will not neutralise the virus (Fulton et al, 2003).
They are prone to ill-thrift, are more susceptible to secondary diseases, and have reduced growth and increased mortality rates (Stokstad and Løken, 2002; Kane et al, 2015). However, many PI cattle can appear completely normal and healthy, living for several years (Presi and Heim, 2010). They can also reproduce normally, although their fertility may be reduced in some cases.
PI dams always give birth to PI calves, further contributing to disease transmission (Lanyon et al, 2014). Semen from PI and TI bulls can also transmit the virus to the dams (González Altamiranda et al, 2012; Read et al, 2020).
If PI cattle are exposed to a CP BVDV strain, or if their own strain mutates to a CP biotype, they will develop mucosal disease (MD) – another severe presentation of BVDV (Lanyon et al, 2014; Peterhans et al, 2010). MD causes ulceration of the mucosal layers of the GI tract, from the oral cavity to the abomasum and intestine. Affected animals tend to be pyretic, anorexic, depressed and have severe diarrhoea, with fresh blood or melena. Mucosal disease is terminal, and most affected PI cattle will die within days to weeks (Lanyon et al, 2014).
BVDV infection in bulls does not seem to affect semen quality (González Altamiranda et al, 2012); however, semen from TI or PI bulls can transmit the virus to dams, potentially impairing fertilisation. Reports are available in the literature of bulls that developed persistent or prolonged testicular infections (PTIs), where the virus is thought to be able to survive in the testes of immunocompetent animals due to the blood-testes barrier (Read et al, 2020).
It is hypothesised that the initial infection in these animals may have occurred around puberty, but the exact PTI pathophysiology is unknown. All documented PTI cases were identified at artificial insemination centres, and the prevalence of such infections in the field remains unclear.
The aforementioned clinical presentations of BVD – acute disease, immunosuppression, reproductive failure, congenital malformations and severe disease manifestations – translate into significant losses at the herd level. BVDV infections are associated with low conception rates, increased calving intervals, decreased growth rates, reduced milk yields, increased somatic cell counts, mortality and culling rates, as well as higher costs associated with the treatment of secondary diseases (Hessman et al, 2009; Houe, 2003; Fountain et al, 2021; Yue et al, 2021a and 2021b). Table 1 summarises the impact that BVDV has on dairy and beef herds.
| Table 1. Negative effects of bovine viral diarrhoea virus infections on dairy and beef herds | |
|---|---|
| Increased | Reduced |
| Calving intervals | Conception rates |
| Somatic cell counts (dairy) | Growth rates |
| Secondary disease (pneumonia, diarrhoea) | Milk yields |
| Abortions, stillbirths | |
| Mortality | |
| Culling rates | |
| Cost of treatments for secondary disease | |
Similar to what happens at the individual animal level, the severity of BVDV infections in a herd will vary depending on the pathogenicity of the infecting strain, the timing of the virus introduction and the level of immunity in the herd (Rodning et al, 2012).
A BVDV outbreak in a naive farm will be much worse than a reinfection in an endemically infected herd or an incursion in a vaccinated herd (Stott et al, 2010; Yue et al, 2021a). This variability is reflected in the estimates of economic losses provided in the literature, which have been reported to range from £0 to £578 per animal in two separate reviews published by Richter et al, and Yarnall and Thrusfield, in 2017. The reported losses tend to be higher in dairy than beef herds (Richter et al, 2017).
BVD vaccines are a valuable tool for mitigating the impacts of BVDV in cattle herds. They have been shown to be effective in improving conception rates and preventing fetal infections, abortions and acute infections (Newcomer et al, 2015; Purtle et al, 2016). However, identifying and removing any PI cattle should always be the first and most important step in controlling BVDV, followed by regular monitoring of the herd status and adhering to strict biosecurity measures and quarantine protocols (Moening and Becher, 2018).
Endemically infected herds, even if vaccinated, will still suffer production losses. Owners of these herds may not perceive that they have an issue with BVD, as the herd may have reached a point where most cattle have acquired natural immunity through exposure to PIs, but new infections will still be occurring.
Young calves, once maternally derived antibodies decline between four and nine months of age (Muñoz-Zanzi et al, 2002), will become susceptible to the virus, and succumb to acute infections and its associated clinical signs until they develop neutralising antibodies. Additionally, any PI cattle present in the herd may have reduced growth rates, can succumb to secondary diseases and MD, and will give birth to more PI calves.
In herds that are free of BVD, vaccines offer protection to minimise the potential consequences of a BVDV incursion – especially in open herds, in nations where the sale of PI cattle is not banned yet, or those farms where cattle may have contact with neighbouring stock. However, as mentioned previously, strict biosecurity measures and quarantine protocols should be prioritised to prevent the virus from entering the herd.
BVDV presents significant challenges to cattle health, welfare and productivity, resulting in substantial economic losses for the cattle industry, both in endemically infected herds or herds at risk of new infections. The variability in clinical presentations, from acute disease to reproductive failures and congenital malformations, underscores the need for comprehensive control and prevention measures.
While vaccines are an effective tool in mitigating the impacts of BVDV, the cornerstone of any control strategy must be the identification and removal of PI cattle, coupled with stringent biosecurity measures and regular herd monitoring. In regions where BVD eradication is not yet compulsory, veterinarians play a crucial role in raising awareness and encouraging proactive measures to prevent and control the virus. By prioritising these strategies, we can improve herd health, reduce economic losses and move closer to the goal of eradicating BVDV from all cattle populations.