21 Apr 2020
David Harwood BVetMed, FRCVS summarises proceedings from the Goat Veterinary Society autumn meeting held in Thirsk, North Yorkshire.

Figure 1. The Goat Veterinary Society (GVS) 40th anniversary dinner. Pictured from left: Ben Dustan, GVS secretary; Daniella Dos Santos, BVA president; Nick Perkins, GVS president; Nick Hart, Sheep Veterinary Society president; Niall Connell, RCVS president; and Bryony Kendall, GVS treasurer.
The Goat Veterinary Society (GVS) marked its 40th anniversary at its autumn meeting with a dinner at The Golden Fleece Hotel in Thirsk, North Yorkshire, attended by RCVS president Niall Connell and BVA president Daniella Dos Santos (Figure 1).
The scientific meeting itself took place at Thirsk Racecourse, preceded by a short annual meeting at which Nick Clayton stood down as treasurer to be replaced by Bryony Kendall. Mr Clayton was thanked for all his hard work in a role he had occupied for many years.
“Abortion and fetal deformity in a small goat herd” – the first paper of the day – was presented by Vanessa Swinson, a veterinary investigation officer (VIO) at the local APHA laboratory.
Her original investigation began with the submission of aborted twin fetuses and placenta from a group of five does – one other doe had also aborted and two others had produced weak, but full-term, kids. No reported problems had been reported in the preceding year.
Postmortem examination (PME) confirmed hydranencephaly in one of the fetuses, raising a suspicion of bluetongue (a notifiable disease) that was subsequently negated. No cerebral abnormalities existed in the second fetus.
Laboratory tests were also undertaken for bovine viral diarrhoea/border disease virus and for Schmallenberg virus – with negative results. No significant bacterial isolates from stomach content existed.

Both brain and placenta were, however, positive to the Toxoplasma gondii PCR test, confirming Toxoplasma as the most likely pathogen, although histological examination of brain tissue confirmed the gross hydranencephaly lesions, but showed no Toxoplasma pathology.
Follow-up blood submissions from adults also confirmed a significant copper deficiency, and one showed a raised Toxoplasma latex agglutination test titre. It also transpired the does were in suboptimal body condition during pregnancy, suggesting overall that the aetiology was likely to be multifactorial.
The second presentation, “Commercial goat world: 40 years and going forward”, was given by Kathleen Wielkopolska of Yorkshire Dairy Goats. She began by highlighting the dairy cow milk overproduction in the 1980s, with milk quotas introduced in 1984.
This stimulated an interest in dairy goat production, with pasteurised goat milk appearing on supermarket shelves in 1985‑6, and the dairy goat sector moved forwards (Figure 2), aided by an increased demand for cow’s milk alternatives. This continued until around 2010, when the demand for goat’s milk levelled.
Foot-and-mouth disease made a huge impact across all farming sectors in 2001. The emergence of BSE and its subsequent identification in goat brain tissue undoubtedly presented a problem, not only on export opportunities, but also on public confidence. Luckily, BSE in goats was an isolated incident and no more cases were identified.
More recently, bTB has emerged as a potential (and real) threat to the commercial dairy goat sector – particularly in those areas where TB is endemic, with goats classified as “spillover” hosts.
Problems have not been restricted to the commercial sector – Escherichia coli O157 made headline news when an outbreak of cases occurred in children after handling goats on an open petting farm. This led to more stringent hygiene measures becoming a mandatory requirement on such units.
The dairy sector will continue to evolve, with extended lactations becoming more widely used; a greater emphasis on goat welfare, behaviour and environmental enrichment; and innovative moves to develop sexed semen, therefore removing the problem of unwanted male kids.
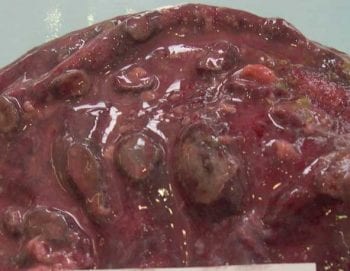
The next speaker was Rudolf Reichel – also a VIO at APHA Thirsk – on “Coxiella burnetii: genome analysis of UK isolates”. Human Q fever cases continue to be reported in the UK, but on a sporadic basis, confirming it as a significant zoonotic infection. The speaker reminded the audience of the devastating outbreak in the Netherlands in which 4,026 human cases were confirmed, with dairy sheep and goats the main source – the reason this disease is kept under constant review.
Survey work in the UK has confirmed a dairy cow bulk milk prevalence of between 22% and 80%. A serology survey in goats in the UK indicated a seroprevalence of 26%. Between January 2010 and May 2019, 63 abortions were attributed to C burnetii, with 33 cattle incidents and 22 goat – of real significance when one considers the substantial difference in overall population sizes (UK figure: 108,000 goats in total).
Q fever infection rarely leads to disease in animals, but may cause abortions – particularly in goats (Figure 3). In humans it may cause flu-like symptoms, including fever, headaches, diarrhoea and vomiting. In severe cases it can lead to pneumonia and hepatitis. Chronic infection is more uncommon, but potentially more severe, and includes heart valve disease.
Genome sequencing data was given using multispacer sequence typing (MST). This ongoing study has shown that, of the small number of isolates tested in the UK, cattle and sheep isolates contained the MST20 genotype (not associated with human disease in Europe), whereas three of four goat isolates contained the MST33 genotype – the one implicated in the Netherlands outbreak. Further UK testing and assessment is required.
“Practical coccidiosis control” by Jo Childs, from Friars Moor Livestock Health, began outlining its impact. This included:
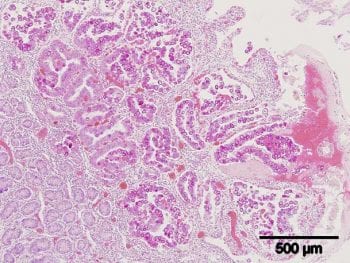
Eight Eimeria species have been recognised in goats, with the pathogenic species recognised as Eimeria caprina, Eimeria ninakohlyakimovae, Eimeria arloingi and Eimeria hirci – reinforcing the importance of speciation and not relying solely on coccidial oocyst counts. The parasite stunts the intestinal villi, therefore reducing the intestinal surface area for water and nutrient absorption (Figure 4).
Clinical signs of acute infection include diarrhoea with blood and mucus, and tenesmus. Chronic and subclinical coccidiosis is characterised by pasty dung, a stary coat, poor growth rates and uneven batches of kids (Figure 5).
A recognised periparturient rise exists in oocyst excretion by pregnant does, compounded by residual infection in the environment and then exacerbated by multiplication through successive batches of kids.
Unlike with nematode burdens, goats will develop some (but not total) immunity to coccidiosis, with a need to achieve a balance between sufficient oocyst exposure to allow immunity to develop without causing clinical disease.
An all in, all out rearing system is the ideal approach, but may not always be practical; the use of oocidal products following cleaning is advised. Products advised for treatment and control included toltrazuril, decoquinate and diclazuril (used under the cascade).

Karin Mueller, a regular contributor to GVS meetings, discussed urolithiasis and its management. Caused by the precipitation of minerals around an organic nidus in the bladder, clinical signs include haematuria, straining and generalised abdominal discomfort with arching of the back.
The condition is seen predominantly in castrated males, with diagnosis based on urinalysis, clinical signs, abdominal palpation, rectal examination of the urethra, and imaging and radiography to locate obstruction. If the bladder or urethra ruptures then sudden relief occurs, followed either by ventral swelling (urethral rupture) then uraemia.
Risk factors include:
Treatment can be medical with the use of muscle relaxants and cystocentesis by the passage of a catheter, although this is problematic due to the anatomy of the urethra or surgical. Surgery treatment may involve the simple removal of the filiform appendage, urethrotomy or laparotomy and tube cystotomy.
Euthanasia must also be an option on welfare grounds if all treatment options have been exhausted.
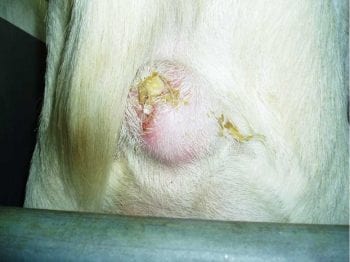
Caseous lymphadenitis (CLA) – the final presentation – came from GVS president Nick Perkins. CLA is a chronic bacterial disease affecting both goats and sheep caused by the facultative anaerobic organism Corynebacterium pseudotuberculosis. It causes a superficial (Figure 6) and visceral lymphadenopathy.
CLA was first identified in goats in the UK in 1990, but was thought to have been introduced earlier by a group of Boer goats imported from Germany in 1987. It is a highly resistant organism in the environment, and can easily survive on fixtures and fittings contaminated with pus from a burst abscess for 50 to 60 days. The main route of entry is via wounds and abrasions from, for example, protruding wire/nails/fence splinters or following excessive pruritus due to ectoparasites.
Borrowing equipment, such as potentially contaminated weigh crates and foot equipment, could inadvertently introduce infection to a clean herd. Following infection, the bacteria are carried to local lymph nodes where replication occurs, resulting in a pyogranulomatous reaction in the target lymph gland. In heavily infected herds, visceral lesions can develop, although these are more common in sheep.
Diagnosis is based on the anatomical association with a local lymph node, by needle biopsy and culture, and by PME in dead or culled goats. An ELISA blood test is available in sheep, but has produced questionable results in goats when used as a screening test. Treatment is problematic since opening and draining an abscess will potentially contaminate the environment, and affected goats may well develop further abscesses elsewhere, so should preferably be separated. A poor response to antibiotic treatment exists generally.