4 Nov 2020
Respiratory and reproductive disease in late summer 2020 is the focus of Axiom Veterinary Laboratories’ latest update.

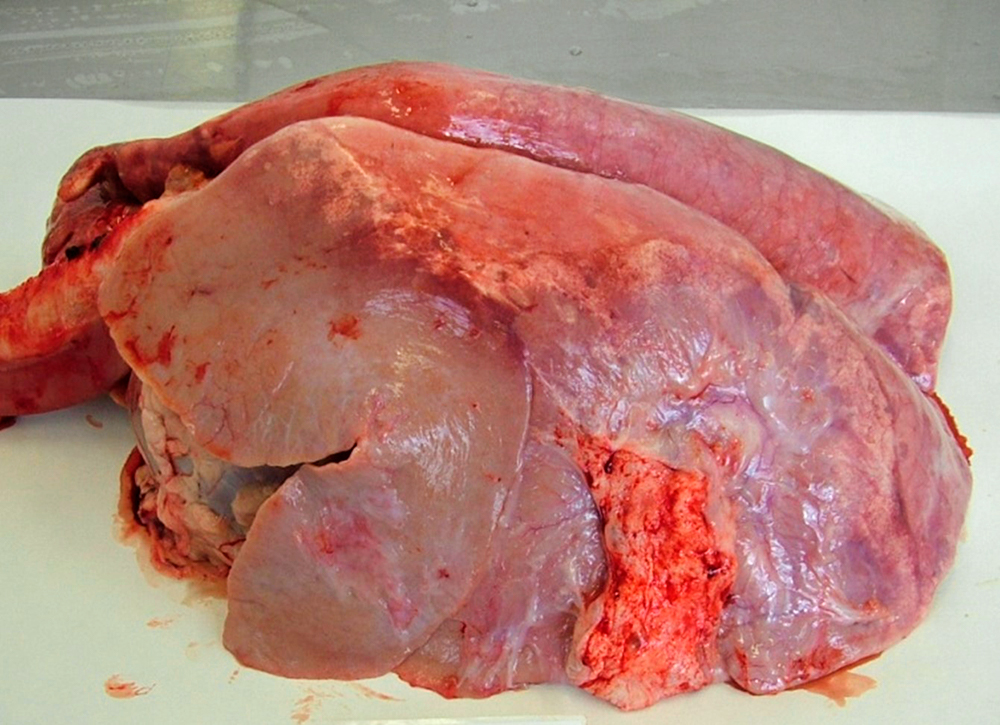
Dictyocaulus viviparus lungworm in a calf.
Presented are selected cases from the ruminant diagnostic caseload of Axiom Veterinary Laboratories.
Axiom provides a farm animal diagnostics service to more than 300 farm and mixed practices across the UK, and receives both clinical and pathological specimens as part of its caseload. The company is grateful to clients for the cases presented in this article.
The focus for this article is gastrointestinal and nutritional disease in early summer 2020.
Lungworm (Dictyocaulus viviparus) was confirmed on Baermann testing in a number of herds and exposure to lungworm was detected on serology.
Cases included a group of weaned heifers exhibiting coughing, mild pyrexia and increased lung sounds on auscultation that had been vaccinated with Huskvac in the spring.
It was also diagnosed on histopathology in a five-month-old dairy stirk. This was one of a group of calves that been coughing three weeks earlier that appeared to respond to anthelmintic, but had since relapsed and had become pyrexic. Viable adults and larvae were found in the trachea and a significant secondary bacterial pneumonia was present. No evidence of nematode death was present following the reported treatment and re-evaluation of the anthelmintic treatment protocol was advised.
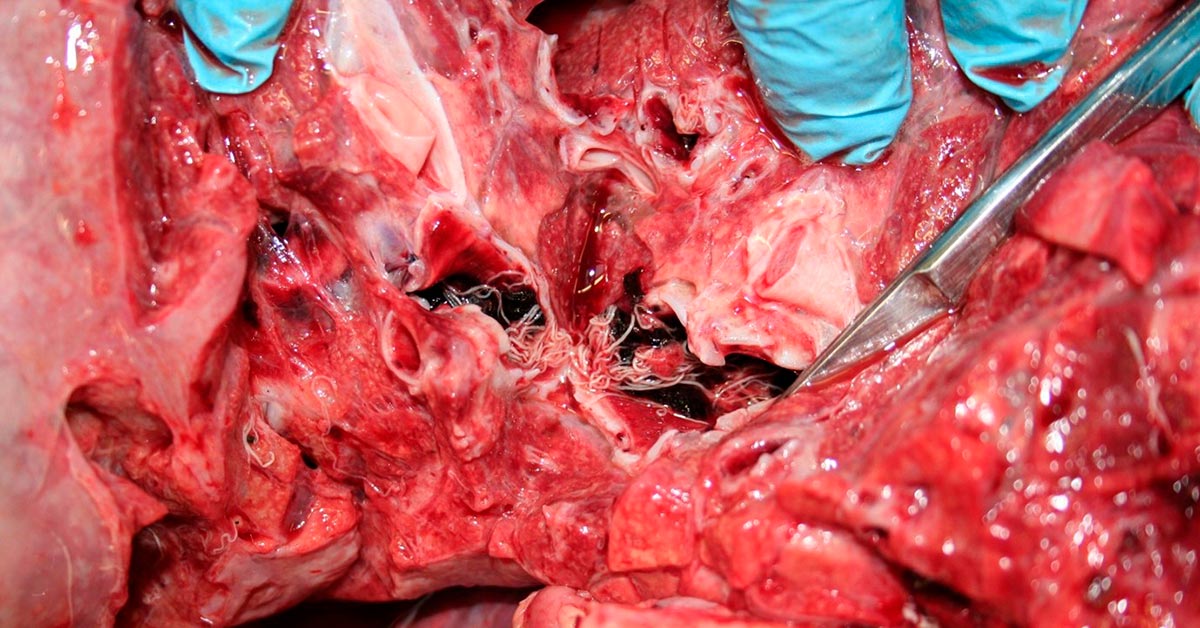
Diagnosis of lungworm is not straightforward, with multiple Baermann tests or serological tests being required to find a positive animal. Issues such as the diagnostic gap in the prepatent period, die-off lungworm larvae in transit and negative results in animals with reinfection syndrome are all confounding factors in diagnostic testing. A very useful summary is available (McLeonard and van Dijk, 2017).
Protostrongylus rufescens larvae were detected on Baermann testing of faeces in two sheep flocks with a history of coughing. Heavy infestations with this lungworm species can be associated with respiratory distress and weight loss. P rufescens also was detected on Baermann testing in a group of coughing adult and kid goats. A strongyle egg count of 3,000epg also was found.
Muellerius capillaris larvae were detected on Baermann testing of a faeces sample from coughing Anglo-Nubian goats. M capillaris is more pathogenic in goats than sheep and may cause signs ranging from a mild cough to a severe cough and dyspnoea.
M capillaris also was detected on Baermann testing in a group of sheep with respiratory signs. M capillaris is typically asymptomatic in sheep, but potentially may predispose to secondary bacterial infections.
Infectious bovine rhinotracheitis was confirmed by PCR in a two-year-old Holstein heifer and by active seroconversion in a pyrexic Holstein-Friesian cow.
Respiratory syncytial virus (RSV) was detected by PCR in the lung of a one-month-old Holstein-Friesian calf, vaccinated against RSV, parainfluenza virus type three (PI3) and Mannheimia haemolytica, and against Mycoplasma bovis with an autogenous vaccine. On postmortem examination (PME), the ventral lungs were consolidated and Trueperella pyogenes was isolated on culture, consistent with a finding of severe serofibrinous bronchointerstitial pneumonia on histopathology, which was likely secondary to RSV infection.
RSV also was detected in one of a number of bought-in calves, housed in the same shed, with respiratory disease and pyrexia.
In a third case, RSV was detected in a recently bought-in 10-month-old weaned suckler heifer calf with open mouth breathing, pyrexia (40.2oC) and muffled lung sounds on auscultation.
PI3 viral RNA was detected by PCR in one of a group of six-week-old calves with increased levels of pneumonia and conjunctivitis.
PI3 or RSV infection also was suspected on histopathology of lungs from two calves in an outbreak of respiratory disease, with the finding of severe bronchointerstitial pneumonia with bronchiolitis fibrosa obliterans. No evidence existed of a bacterial involvement, including M bovis.
Rising titres to M bovis were detected in a group of 60 10-week-old to 14-week-old heifers with signs of pneumonia – including dyspnoea, pyrexia, nasal discharge and coughing – with 33% morbidity and nil mortality.
High positive titres to M bovis, suggestive of recent exposure, were seen in calves in several herds, including in a six-month-old Belgian Blue heifer calf that was dull, pyrexic and had increased lung sounds.
M bovis was detected by PCR on a nasopharyngeal swab and high positive titres were present in five out of six animals sampled for serology in a group of 18-month-old to 2-year-old beef finishers in an ongoing pneumonia outbreak.
A number of cases of bacterial bronchopneumonia were diagnosed, primarily on histopathology. Examples include:
Organisms such as Pasteurella multocida, M haemolytica, Histophilus somni and, in younger calves, Escherichia coli may be implicated; and where minimal involvement of the airways and alveolar parenchyma exists, haematogenous spread of infection is likely.
Additionally, no evidence existed of underlying viral infection, lungworm or aspiration pneumonia in these cases – and it is likely the bacteria acted as primary pathogens, which can occur with periods of stress, management change, adverse environmental conditions and concurrent immunosuppressive disease (for example, bovine viral diarrhoea [BVD] and tick-borne fever).
Pasteurellosis was diagnosed on histopathology in a four-month-old lamb, with the finding of severe, necrotising, suppurative pneumonia with intralesional bacterial colonies. This was one of three lambs to die in one week after just receiving the second Pasteurella/clostridia vaccination, so they would have still been developing immunity.
In a second case, a preweaned lamb dropped dead two weeks after its first dose of vaccine. Lung lesions were found on PME and M haemolytica was isolated from lung. However, the isolation of Erysipelothrix rhusiopathiae – also from the lung – was unusual. Typically in the UK, this soil-borne opportunistic pathogen is associated with arthritis in lambs, but occasional reports exist of endocarditis and pneumonia associated with infection, and it may have been a secondary pathogen in this case.
In a third case, histopathological findings consistent with pasteurellosis were found in the lungs of a dead lamb with cranial lung lobe consolidation on PME.
Salmonella enterica subspecies enterica serovar Dublin (S Dublin) was isolated from the lung of a five-week-old Holstein-Friesian calf that died suddenly and had pulmonary consolidation on PME. S Dublin is known to cause pneumonia as well as enteritis, particularly in dairy calves.
Atypical pneumonia was diagnosed on histopathology in a vaccinated flock with recent lamb losses. This is most common in post-weaned lambs where ill thrift and mild respiratory signs are seen. The condition is multifactorial and commonly associated with P multocida, M haemolytica, Bibersteinia trehalosi, staphylococci and streptococci. Additionally, Mycoplasma ovipneumoniae is frequently found and is thought to be a significant contributing factor to the pathology. In this particular case, a significant suppurative component was present, which would have accounted for the death of the lamb.
Similar lung pathology was found in a five-month-old lamb that had died acutely and was found to have pulmonary abscessation on PME; and in a five-month-old goat kid, one of four to die acutely, with cranioventral pulmonary consolidation found on PME.
In a fourth case involving a three-month-old lamb, bacterial pericarditis was also present, which may have reflected progression of bacterial infection from the lungs to septicaemia, or due to septicaemia arising from another source.
Staphylococcus aureus was isolated in pure growth from the lung of a four-month-old lamb with marked pulmonary congestion, potentially consistent with staphylococcal septicaemia. The lamb also had a high in-house worm egg count and concurrent disease may have increased susceptibility to a staphylococcal infection. No ticks had been found on the lamb.
Bronchopneumonia of multifactorial aetiology was diagnosed in one of a number of five-month-old Holstein calves to die acutely.
Histopathology detected two phases to the pneumonia. A more chronic active pneumonia with bronchiolitis fibrosa obliterans was suggestive of pneumotropic viral infection followed by secondary bacterial pneumonia. Not surprisingly, due to the chronicity of the lesions, viral PCR was unrewarding. Significant plasmalymphocytic peri-airway infiltrate was also present, raising the possibility of concurrent mycoplasmal infection, and M bovis was detected on PCR. Other sections showed more acute change indicative of a bacterial bronchopneumonia.
This most likely arose following a flare-up of infection with endobronchial spread as a result of stress, management changes and concurrent immunosuppressive disease. The isolation of H somni and P multocida was likely to be significant in this respect.
Ovine pulmonary adenocarcinoma (OPA) was diagnosed in eight flocks, with ill thrift a common finding.
In one flock, in which OPA had been diagnosed for the first time a week previously, it was confirmed on histopathology of lung from two of three recent deaths, all of which had lesions grossly suggestive of OPA. In the third sheep, the lesions were associated with the lungworm M capillaris.
In another flock, one ewe was reported to have exhibited rapid weight loss, progressing to respiratory distress with a positive wheelbarrow test.
Secondary bacterial pneumonia was seen in association with OPA lesions in several flocks – a relatively common finding in cases of OPA, which often predisposes to acute clinical disease.
Positive titres consistent with maedi-visna infection were found both on flock screens and in sheep with clinical signs potentially consistent with maedi-visna, particularly ill thrift.
Pulmonary thromboembolism secondary to hepatic abscessation was diagnosed in a two-year-old Ayrshire heifer that died just before calving. Five abscesses – with one measuring 15cm in diameter – were found on PME, the lungs appeared oedematous and acute septic pulmonary thromboembolism was diagnosed on histopathology.
Rupture of hepatic abscesses into the caudal vena cava can lead to rapid endotoxaemia, release of contents into the circulation and entrapment within the lungs, leading to pulmonary oedema, haemorrhage and congestion. Normally a rapid onset of respiratory signs or sudden death occurs and, in some cases, haemoptysis following major vessel rupture due to pulmonary arteritis.
Liver abscesses are normally secondary to ruminal acidosis, and re-evaluation of the die and feeding management was advised.
Severe proliferative interstitial pneumonia consistent with fog fever was diagnosed on histopathology in a three-year-old Hereford cow that died 24 hours after developing signs of acute respiratory distress.
A number of fetuses in different herds were positive on PCR for Neospora, consistent with infection, although histopathology on heart and ideally brain is required to confirm neosporosis as the cause of abortion.
In one case in a dairy herd, where three abortions had occurred over a two-week period, E coli also was isolated in pure growth from fetal stomach contents, although typically this is only a cause of sporadic abortions in cattle.
In a second herd, after the diagnosis of Neospora abortion in a fetus, seven more aborted and six of these had positive titres to Neospora. Such an abortion storm is typically seen with an exogenous source of infection following ingestion of oocysts shed in dog faeces.
Neosporosis was also suspected to be the cause of abortion in a three-year-old Holstein-cross cow that aborted at four-and-a-half months gestation, with the finding of a non-suppurative myocarditis on histopathology. The herd had a history of Neospora abortion and infectious bovine rhinotracheitis infection in heifers, and five abortions had occurred in the previous three months.
Five out of 8 ewes seroconverted to Toxoplasma in a flock of 500 that lambed in April and May, in which 2% had been barren and 2% had aborted.
In a second flock, high positive titres were detected in all 6 ewes sampled in a flock where 2 out of 30 sheep had aborted and 2 were barren at scanning.
In a further flock of 170 ewes, where 10% had been barren and 15% had aborted, 7 out of 8 ewes were antibody positive for Toxoplasma.
BVD viraemia was confirmed in a fetus aborted at seven months in a dairy herd, consistent with in utero infection with BVD. Neospora also was detected by PCR, but confirmatory histopathology was not carried out.
In a second herd, two aborting heifers in a dairy herd were confirmed as BVD viraemic; further testing was advised to determine whether these were transiently or persistently infected animals.
S Dublin was isolated from the fetal stomach contents of a fetus aborted by a Holstein-Friesian cow three weeks off calving. This was the second cow to abort in three days and the cow was unwell.
S Dublin was also the cause of abortion in a Holstein cow that aborted one month off calving in another herd.
In a third case, S Dublin was the cause of abortion in a dairy cow that aborted at seven months’ gestation; the cotyledons were necrotic and odoriferous, bloody fluid was present in the abdomen of the fetus.
S Dublin also was isolated from the stomach contents of a rotten fetus, the second abortion in a Holstein-Friesian herd in which two abortions had occurred in quick succession, and in a fifth dairy herd with a history of abortions.
Yersinia pseudotuberculosis was isolated from the stomach contents of a fetus aborted by a Holstein-Friesian heifer, the second to abort over a short period in a block calving herd.
This organism may be found as a gut commensal in healthy cattle, and wild birds and rodents are reservoirs of infection. It tends to cause sporadic abortion in domestic ruminants.
Streptococcus lutetiensis was isolated from the fetal stomach contents of an aborted bovine fetus. This has been associated with abortion in small ruminants; therefore, it may have been significant in this case of bovine abortion.
Serratia liquefaciens was isolated from the stomach contents of a fetus aborted at five months by a Holstein cow.
This is an environmental organism causing, for example, mastitis in cattle, and its isolation in pure growth may have been consistent with abortion secondary to an ascending infection of the urogenital tract.
Placenta was submitted from a Friesian herd as part of an ongoing investigation into abortion. The calf had been born alive, but very dry, and the placenta was thick and leathery. Histopathology detected severe necrotising placentitis with intralesional bacteria most typical of Bacillus licheniformis.
Three cows that had calved early in a dairy herd seroconverted to Q fever, one with a high titre suggestive of recent exposure. A known tick issue existed on farm and positive titres to Q fever had been observed the previous year.
Although Q fever is known to cause abortion storms, particularly in goats, it only rarely causes sporadic abortions in cattle. Abortions are characterised by placentitis and Coxiella burnetii PCR on placenta can be used to confirm infection.
Dystocia was diagnosed on histopathology of a Belgian blue calf that was found dead during a caesarian despite no straining being observed in the dam.
Widespread haemorrhage was noted within numerous organs, most suggestive of hypoxic and agonal events, and multifocal myocardial necrosis was present that may have been the result of hypoxia and excess catecholamine release, the latter associated with severe fetal stress. This was supported by the presence of meconium within airways, which is a common finding in aborted and stillborn fetuses associated with hypoxia and stress.
Several cases of Klebsiella pneumoniae mastitis were seen in herds over the late summer.
Klebsiella species are environmental Gram-negative organisms and outbreaks have been associated with sawdust bedding. It can also cause a severe toxic mastitis.
Among the more unusual bovine mastitis isolates were:
S aureus was isolated from one of four milk samples submitted from an Anglo-Nubian herd with high cell counts. This is a contagious mastitis pathogen, which often causes a chronic, subclinical mastitis with occasional flare-ups of clinical mastitis. Sporadically infected animals present with an acute, severe, gangrenous mastitis.
Infection by S aureus usually causes a fluctuating high cell count. Infected udders and damaged teat skin are the main reservoir of S aureus; therefore, infected goats are the main source of infection. Transfer occurs mainly by indirect spread during milking.