4 Apr 2016
Ian Nanjiani focuses on available treatment options, with guidance on how to get the best out of them by targeting the correct life cycle stages at the right time of year.
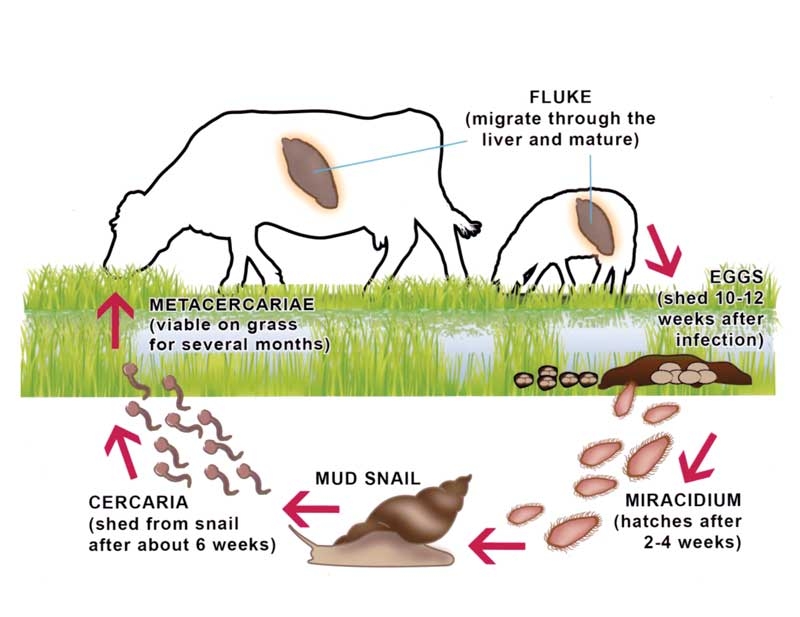
Figure 1. Liver fluke life cycle. (from COWS Technical Manual For Veterinarians and Advisors: Liver Fluke, 2013).
Fasciola hepatica requires an intermediate host (the mud-snail Galba truncatula) to complete the ruminant infection cycle (Figure 1)1. The snails have fairly specific requirements, needing water, warmth and nutrients provided by algae they consume in slow moving water, so are commonly found at the edges of standing bodies of water, streams, or land poached by machinery or livestock.

Temperature and moisture affect the multiplication of both the snail and the parasite’s infectious stages (the newly hatched miracidia, which infect the snail, and the metacercariae on the herbage, which infect or reinfect the host).
Temperatures of 10°C or above allow snail and parasite multiplication, and the capacity of a snail to produce approximately 100,000 offspring in a three to four-month period2 – coupled with a 500-fold parasite multiplication within the snail3 – can lead to substantial pasture metacercarial loads in relatively short periods of time. This is particularly the case in warmer, wet weather when both the snail and the parasite multiply much more rapidly.
Following ingestion by the host (in our case, the grazing ruminant, although other wildlife – including man – are susceptible to infection), the metacercariae hatch and immature fluke migrate through the gut wall to the liver. The immature fluke take 10 to 12 weeks to reach the bile ducts, where they lay their eggs, so the life cycle is completed in around 17 to 19 weeks. Adults survive in sheep for a number of years, although adult loads in cattle may reduce with time as the bile duct solidifies, preventing blood feeding.
Historically, the May to October period has provided the necessary temperature and moisture characteristics for fluke multiplication, although milder winters lately have extended this period – increasing the period of infection risk, allowing better pasture survival on infective stages and increasing the likelihood of early snail and animal infection in subsequent seasons. Current global warming predictions point to an increasing risk period and an expansion of suitable snail habitats, and warm, wet summers represent the highest risk periods for acute infection.
Acute disease occurs following parenchymal damage caused by mass migration of immature stages and can be rapidly fatal in sheep (primarily through blood loss). Acute disease is rare in cattle, though – perhaps due to their larger, tougher liver. The syndrome is generally preceded by a warm, wet spell encouraging high pasture metacercarial loads, and treatment of acute infection must therefore target the immature parasite stages.
Subacute and chronic disease is common in both sheep and cattle later in the grazing season and into the housing season, with animals classically showing loss of condition, submandibular oedema (bottle-jaw), anaemia and a predisposition to some clostridial diseases. Additionally, degrees of immunosuppression (sufficient to interfere with the tuberculin test) have been reported by some authors, with implications for statutory TB testing. Subclinical infection has been implicated in reduced production in terms of growth, fertility and milk production.
Fasciolosis is an “iceberg” disease – visible losses are only part of the full impact, and animals don’t appear to develop protective immunity following infection. Lower fluke burdens may still affect growth rates, carcase composition, fertility and, in dairy cattle, reduced milk yields and changes in milk quality without any external signs of disease. Annual losses to the industry attributable to fasciolosis (liver condemnation and lost production in England) have been estimated to be £13 million to £15 million (beef and sheep) and £23 million (cattle)4.

In England in 2012, more than 16% of cattle livers (259,000) and nearly 7% of sheep livers (582,000) were excluded from the human food chain due to liver fluke infestation5, similar to levels described by other authors6. This highlight the importance of robust investigation of ill-thrift on farms, and the value of postmortem data, either from disease investigations, or from abattoir feedback.
The same general principles of good practice apply to flukicides as all medicines for food-producing animals, and are summarised below.

Triclabendazole (TBZ) resistance has been reported in the UK6,11-13, so its use should ideally be reserved for the treatment of acute disease in sheep (and rarely cattle). The faecal egg count reduction test has been used to detect TBZ resistance and appears to function well enough for field use in the absence of validated in vitro methods, provided sufficient animals are tested7,8.
Applying these general treatment principles to each farm requires a detailed understanding of its stocking and grazing patterns, and control of liver fluke disease should be an important part of a farm health plan drawn up with the farmer’s veterinary surgeon, as discussed in this publication (Nanjiani, 201515).
Monitoring the levels of infection in sheep and cattle using fluke egg counts, bulk milk antibody, abattoir returns and veterinary investigation of ill-thrifty animals is essential to successful control, as is assessing the fluke risk of the pastures – using as accurate a fluke risk forecast as is available. NADIS provides roughly correct regional forecasts based on rainfall and temperature, which can be translated into farm/pasture risk in discussion with the farmer.
Recent research from the EU-funded GLOWORM project has developed mathematical models that attempt to accurately predict increased metacercarial abundance/infection risk, so more accurate prediction systems will hopefully appear in the near future16.
A variety of treatment scenarios and therapeutic options exist (Tables 1 and 2), which are either intervention treatments (to safeguard health and welfare) or strategic (group) treatments to minimise pasture contamination.
Production benefits have been demonstrated treating infected animals before overt clinical signs develop17, which is logical given the damage F hepatica causes the liver.
The basic fluke treatment approach in endemic farms comprises:
Given the warm, wet winter of 2015/2016, we can expect an increased risk of fluke this year:
As for so many parasites, the treatment of F hepatica is not a straightforward affair, with the parasite expanding its footprint and probably its risk period in the UK. Our product armoury is limited (particularly so for dairy cattle) – products have no persistent effect, resistance is reported, and livestock will generally show signs of clinical disease only when substantial liver damage has occurred.
The complex epidemiology means no “one-size-fits-all” treatment regimen can be applied to all farms. This article has focused on treatment, but it is integrated parasite control using a combination of risk-based grazing, close monitoring of stock (maximising all data sources including bulk milk, FECs, abattoir feedback) and strategic product use informed by climatic fluke prediction models that provides our best bet for on-farm control.
It’s time to sit with our clients and develop integrated control plans for all major parasites – we can’t just keep hitting the bottle.