7 May 2018
Sotirios Karvountzis examines up-to-date applications of bovine endoscopy, its advantages, concerns and available training options.
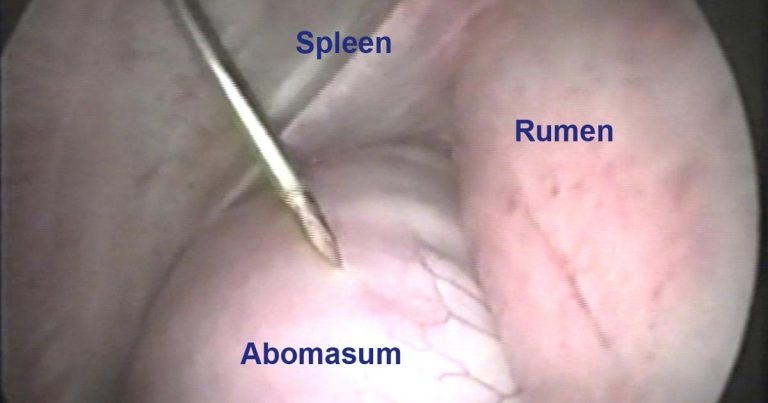
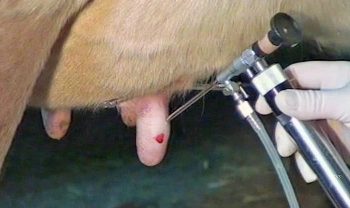
Figure 1. Endoscopic image during abomasocentesis of a left displacement of the abomasum. Image: Sotirios Karvountzis
In human abdominal surgery, most explorative and corrective procedures are carried out endoscopically. Endoscopy has become part of normal canine, feline and equine practice, but in cattle, the procedure has some way to go.
Correction of left displacement of the abomasum (LDA) is, by far, the most popular application of endoscopy, whereby right paramedian abomasopexy takes place. This corrective technique has four variations:
In all techniques postoperative antibiotic treatments are not usually required unless concurrent disease is present (Figure 1).
Correction of the right displacement of abomasum (RDA) is treated by a similar technique to the two-step LDA correction (Janowitz, 1998). Non-volvulated cases are amended with relative ease, whereas volvulated ones have a varying level of complexity. It is worth noting volvulated cases require two surgeries 12 hours apart – the first to deflate and reposition the displaced abomasum, and the second to carry out the abomasopexy. Between the two visits, rehydration with large volumes of fluids is advised. Finally, endoscopic corrections of torsions of more than 360° are challenging.
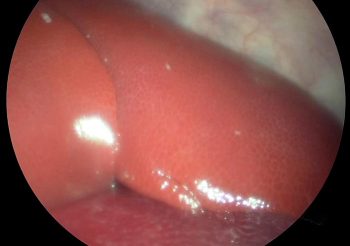
Exploratory laparoscopy is the second most popular endoscopic application and highlights the limited diagnostic means cattle clinicians have at their disposal beyond the thermometer, stethoscope and their clinical experience. With minimal intrusion from the left, ventral or right side of the animal, we can diagnose abdominal ailments that, otherwise, would be left to anecdote. Crucially, it offers the vet an irreplaceable prognostic tool. For example, while the patient is fit for human consumption, in cases of poor prognosis, the animal can be slaughtered before any treatment commences. This, in turn, reduces antibiotic use and prevents unnecessary suffering (Figures 2 and 3).
The pulmonary content consists primarily of air, which is not echogenic in ultrasound examinations. Although ultrasound inspection of the chest should be used before endoscopy, exploratory thoracoscopy complements diagnostic ultrasound in two ways:
The key concern about thoracoscopy is the inadvertent induction of pneumothorax and a clear plan to prevent or minimise it is required before embarking on this procedure.
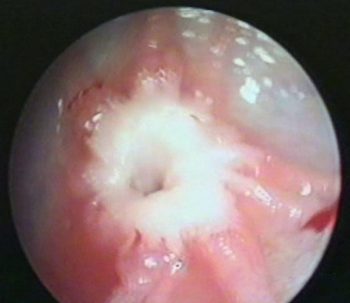
Theloscopy, or teat endoscopy, is a minimally intrusive exploratory and therapeutic technique to treat stenosis of the teat orifice, blockage of the teat cistern and injury of the teat wall (Ebner, 2009). Theloscopy allows the clinician to handle teat problems with minimal invasion, while helping to reinstate milk flow, milk yield and somatic cell count of the affected quarter (Geishauser et al, 2004). Following treatment, patients will experience a slightly increased udder somatic cell count (Figures 4 and 5).
When conservative hormonal treatment of the ovarian cyst fails, aspiration of the cystic content can be carried out under the guidance of ultrasound or endoscopy. Endoscopic correction takes place with two portals placed on the left paralumbar fossa while the animal is standing. An assistant is required to carry out a rectal examination of the patient and present the offending ovary to the endoscopic surgeon. Anecdotally, chronically cystic ovaries following aspiration of their content suffer a high rate of relapse, regardless of corrective technique (Figure 6).
A version of exploratory laparoscopy can be used as a diagnostic tool in calves suffering with umbilical hernia prior to laparotomic surgery. The aim of the technique is to determine the location and extent of adhesions implicating the omentum and the hernial sac, before the commencement of corrective surgery.
Endoscopic explorations and corrections permit the surgeon to fully visualise the target organs (Babkine and Desrochers, Babkine et al, 2005; 2006; Bouré, 2005), whereas it is not possible with other techniques (Newman et al, 2008), such as Grymer-Sterner. As the surgeon is able to handle sharp instruments internally with confidence, the risk of contiguous visceral trauma is drastically reduced. For corrective techniques, a single abomasocentesis is required – the deflation is passive and, in the author’s opinion, the abomasal decompression achieved is higher than in other bloodless techniques.
Following abomasocentesis, localised peritonitis is established, but without any bacteria present, despite the possible leakage of abomasal content (Wittek et al, 2012). For all the reasons mentioned, patients display quicker recovery compared to controls, as some of the aforementioned studies have indicated. In the author’s opinion, herd keepers favour this technique because the animal can rejoin the herd at the first postoperative milking.
Strong evidence exists that endoscopically corrected LDA cases produce more milk postoperatively than laparotomically ameliorated ones. When Seeger et al (2006) compared subsequent milk yields, between endoscopic abomasopexy by Janowitz and laparotomic omentopexy by Dirksen, they found strong evidence of the Janowitz group producing 2.5 litres of milk per cow per day more than the Dirksen one for up to six weeks postoperatively (P <0.01). Beyond this period, yields between the two groups did not present any significant difference – a finding also corroborated by Wittek et al, 2009.
With the exception of thoracoscopic ones, as a result of their small size (the largest being 1.5cm long) portals do not require suturing postoperatively, due to the low risk of infection. Incision-related complications at the portals or the abomasopexy point seldom occur, including those located ventrally at a relatively dirty area of the body (Fubini et al, 1992). In normal circumstances, endoscopic operations require less systemic antibiotics compared to laparotomic corrections (Roy et al, 2008).
Other than complicated cases, the author advises no course of antibiotics to be administered following the procedure. Beyond surgical cases, considerable welfare and economic implications exist for clinicians if they can decide, prior to treatment, whether an antibiotic course would be beneficial or if it has a substantial chance of success. This is very often achieved with exploratory endoscopy; therefore, we can conclude the application of exploratory and corrective endoscopy reduces antibiotic use.
The procedure is not physically strenuous for the surgeon, nor is it unsafe – particularly when the animal’s back legs are shackled. Also, the operation is not demanding on farm personnel as, for the standing techniques, only one assistant is required to help restrain the patient.
Finally, postoperative fertility and survival of corrective endoscopy are comparable to those of other laparoscopic techniques (Seeger et al, 2006; Wittek et al, 2009).
As passionate as one can be about a procedure abundant with advantages, equally one has to be brutally honest about its concerns – albeit a few.
The level of investment is high and is similar to the cost of a linear probe scanner. The endoscopic kit may be used as often as a scanner, but not every member of the practice has one in his or her car. This creates various management considerations, which are further discussed later. Some practices in the Netherlands and Germany equip all their large animal clinicians with endoscopic kits, but these practices are the exception rather than the rule.
A set sequence of events exists in any endoscopic application. For example, an LDA correction consists of preparation, setting up the optical portal, setting up the working portal, abomasocentesis and abomasopexy. It is interesting to note the first two steps are the same for an exploratory laparoscopy. The procedure is modular and easy to teach, but some find it takes time to become confident with the method, as well as familiarise themselves with the contents of the kit. For example, five sets of trocars and cannulas will be in use and, additionally, three more instruments are regularly used (Figure 7). The average trainee would need 10 cases before feeling fully confident, whereas with laparotomic approaches or bloodless corrections, this number may only be 5.
The risk of visceral trauma is low, as most steps of the procedure are done under endoscopic observation. Any eventualities are predictable, and trainees are encouraged to identify them and work under a standard operating procedure. For example, during abomasopexy, as the abomasum is fixed in the ventral right abdominal wall and caudally to the xiphoid process, a risk of milk vein injury exists. A simple glance externally at the area where fixation takes place, and adjusting the location of the instruments accordingly, is enough to prevent any problems. It is worth mentioning the overall risk of milk vein injury with endoscopy is similar to laparotomic paramedian corrective techniques.
The 5mm and 8mm trocar and cannula are one of the many multifunctional and versatile contents of the kit. Care needs to be taken to avoid losing the nut that holds the valves in place. A simple check to make sure the nuts are tight in-situ before they are used would suffice. All the instruments are made from stainless steel; however, particularly long tools, such as the Christiansen spieker, if forced excessively, may bend slightly. Gentle handling of the instruments, therefore, would be adequate to prevent this issue from happening.
With the exception of the endoscope, the remaining metallic instruments can be cold sterilised or autoclaved. Special care must be taken to prevent damage to the very expensive optic fibre of the endoscope. This is because most disinfectants, such as iodine povidone or chlorhexidine gluconate 4% w/v, are harmful to optic components. Use of recommended liquid pH-neutral cleaner products can address this effortlessly.
Finally, the rechargeable light source – although shrouded in a purpose-built protective cover – is not waterproof. Care must be taken not to submerge it in water, as this will cause damage and reduce its luminosity.

If the practice owns just one kit, while various surgeons are trained to carry out the procedure, the endoscopic set needs to be managed efficiently between the surgeons. Furthermore, should additional visits be required before a patient is seen, this has to be reflected in the business plan. Likewise, the set has to be managed at a multi-branch practice where it is shared between a number of centres; however, the latter is simply addressed by allocating a set per major branch.
The duration of endoscopic corrections – as expressed by the length of time spent between arrival and departure from the holding – is an average of 44 minutes. This is more than the average Grymer-Sterner duration of 28 minutes and less than the average duration of the standing laparotomic corrections of 83 minutes. It is evident, by introducing endoscopy on the back of laparotomic corrections, time on farm is halved, but the same charge as before for the procedure can be issued. This would adequately cover for any additional visits required, while it offers the added option of sick cow explorations.
Finally, rapid cold sterilisation between visits allows the instrument set to be used multiple times before it is returned to the practice.
If you are unsure about the concept and would like to be convinced, the course “Introduction to bovine endoscopy” might be for you. This is a one-day course where a maximum of 12 delegates, split into three groups, experience endoscopic applications in-vitro and with visual aids.
If you are serious about investing in the technique, cost-effective on-site training is available at your practice. The author will join your practice for a minimum of five days to train team members in the technique. This in-situ approach allows a “wide net” to be spread when looking for suitable cases to treat. You’ll receive bespoke advice, particularly about your day-to-day management of the endoscopic kit.
For more information, visit https://mendipvets.net or www.facebook.com/mendipvets