11 Sept 2017
Monitoring and evaluating possible infestations and control measures is vital, says Mike Taylor.

Figure 1. Worm and fluke infections in adult dairy cattle need to be carefully monitored, so the effectiveness of control measures can be evaluated.
Gastrointestinal nematode infections that cause parasitic gastroenteritis (PGE) are most commonly encountered in weaned calves, and usually predominate in the second half of their first grazing season.
Abomasal nematodes are generally considered to be the most pathogenic, of which the most predominant and economically important species is Ostertagia ostertagi. Immunity to disease takes a while to develop, and cattle are not normally considered to be immune until they have experienced two complete grazing seasons.
The immunity is not sterile, and adult cattle can frequently harbour O ostertagi infections, which, in some individuals, may result in reduced performance. In younger cattle, it has been shown subclinical nematode infections can lead to reduced appetite and feed intake (Fox, 1997), and to a marked reduction in daily grazing time associated with a reduction in herbage intake and consequent production losses (Forbes et al, 2000).
Cooperia species, found in the small intestines, are very common in young cattle in their first grazing season, and are the main contributor to faecal worm egg counts, particularly where treatment failures associated with macrocyclic lactone (ML) anthelmintics are suspected.
The main species found in the UK is C oncophora, which is generally considered to be a mild pathogen in calves, although in some studies it has been associated with loss of appetite and poor weight gain (Coop et al, 1979).
Occasionally, a heavy infection can induce intermittent diarrhoea. Cattle appear to mount a rapid immune response to this parasite, and associated faecal egg counts (FECs) tend to decline towards the end of the first grazing season and remain low in subsequent years.
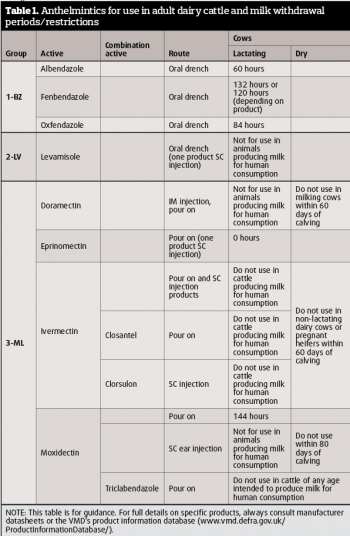
The choice of wormers available for both the treatment and prevention of cattle parasitic helminth infections is in Table 1. Products are marketed primarily for use in worming strategies for the control of parasitic nematode infections in cattle that generally target first-year grazing calves. Increasingly, though, they may be applied to cattle in their second grazing season, and, for some, for use in adult dairy cattle to improve milk production.
For first-year grazers, several highly successful control and treatment strategies are available, combined with a range of application methods that include pour-ons and boluses, as well as more conventional injections and oral drenches (Taylor, 2004). For these animals, worming strategies have proved successful, particularly since the launch of the 3-ML class of anthelmintics, which now dominate the cattle “wormer” market.
Individual product activity and persistence against reinfection varies with the different compounds, and with the formulation and method of application. A survey of parasite control methods, on beef farms in south-west England, showed topical treatment (predominantly the use of ML pour-on products) was the most common method of anthelmintic administration (Barton et al, 2006).
Many of these worming products, however, cannot be used in milking cattle, due to restrictions on use in cows producing milk for human consumption, or in cattle during the dry period, including pregnant heifers prior to calving. Choice is, therefore, limited to eprinomectin, which has a zero-milk withdrawal period, and to a small number of benzimidazoles (1-BZ) products (containing albendazole, fenbendazole or oxfendazole) and two moxidectin pour-on products, which have prescribed milk withdrawal periods (Table 1), during which time milk will need to be discarded.
Dairy cows are known to be infected with small numbers of gastrointestinal nematodes (Agneessens et al, 2000; Borgsteede et al, 2000), but may, nevertheless, show production responses to anthelmintic treatment (Gross et al, 1999).
Studies with eprinomectin have shown milk yield responses of around 1kg/day following treatment during lactation (Nodtvedt et al, 2002; Reist et al, 2002) and improved fertility when treatment was given at calving (Sanchez et al, 2002).
Heifers show a particularly marked treatment response, which possibly reflects their relative immaturity and greater susceptibility to gastrointestinal nematodes (Forbes et al, 2004).
Monitoring worm infections in adult dairy cattle can aid evaluation of the effectiveness of worm control measures and to target anthelmintic treatments where required (Figure 1). Assessment of worm infections at the herd level can be attempted using one, or several, different diagnostic methods. However, neither FECs nor plasma pepsinogen values have been shown to provide quantitative measures of worm burdens, or their impact, in adult cattle (Vercruysse and Claerebout, 2001).
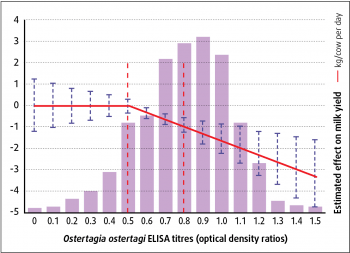
The method generally recommended is to base treatment on the determination of parasite antibodies by bulk milk tank ELISA. Several studies that investigated the correlation between O ostertagi and liver fluke (Fasciola hepatica) infections measured by antibody titres and the herd’s milk yield. Optical density ratios (ODR) have been used to determine threshold levels, above which productivity is negatively affected and economic losses are estimated (Figure 2).
It is suggested anthelmintic treatments in adult milking cows should be targeted at herds with a high worm challenge and reduced productivity. The bulk milk tank ELISA test can be used as a guide to identify dairy herds with potential to increase milk yield in response to anthelmintic treatment.
The most appropriate time to collect bulk tank milk samples is generally in the autumn, prior to housing. A positive treatment response can occur with ODR values greater than the cut-off point of 0.5. However, more reliable responses in milk yield generally occur in herds with an ODR value of 0.8 or more.
Results should be interpreted based on farm management factors, previous worming treatments and the risks from other parasites. It is worth noting results may vary between different laboratories and should be interpreted in accordance with guidelines provided by the laboratory concerned.
The lungworm Dictyocaulus viviparus is commonly encountered in first-year grazing animals in summer or early autumn, but is also increasingly reported in older animals, including dairy cows. Lungworm infection is characterised by bronchitis and pneumonia and typically affects young cattle during their first grazing season on permanent or semi-permanent pastures.
Outbreaks of disease occur from June until November, but are most common from July until September. Parasitic bronchitis may also be seen in adult cattle as a herd phenomenon, or in a particular age group within a herd, if animals have failed to acquire immunity through natural challenge in earlier years. Such animals may develop the disease if they are exposed to heavy larval challenge, as might occur on pasture recently vacated by calves suffering from clinical husk. In addition to coughing and tachypnoea, a reduction in milk yield in dairy cows is a common presenting sign.
The same anthelmintic choices discussed already for the control of gastrointestinal nematodes apply equally to lungworm treatment. However, the severity of lungworm disease may necessitate additional supportive treatment.
Patent lungworm infections occur in adult cattle and are diagnosed, using the Baermann method, by the detection of first-stage larvae in faeces.
Like the situation with gastrointestinal nematodes, several lungworm ELISAs have been developed to detect lungworm-specific antibodies. Most are based on the use of native lungworm antigen, but more recently recombinant proteins have been developed for use in commercialised “dipstick” ELISAs, such as the Ceditest lungworm ELISA (Cedi-Diagnostics, Lelystad, the Netherlands).
The presence of antibody indicates exposure, but not necessarily active infection or immunity to the disease. As such, ELISA results used to diagnose infection in individual animals must be interpreted with care and it is more appropriate to submit a representative number of blood samples from a suspect herd.
Several positive results provide an indication of herd exposure, plus the need for further investigations and/or possible treatment. A bulk milk ELISA has also been developed and offers the potential for a cost-effective method for epidemiological studies and herd monitoring programmes.
Liver fluke disease, or fasciolosis, is due to the trematode parasite Fasciola hepatica. Fasciolosis arises from the migration of large numbers of immature fluke through the liver, or more usually in cattle from the presence of adult fluke in the bile ducts – or both.
Liver fluke can infect all grazing animals, but they mainly affect sheep and cattle, and are generally less pathogenic in cattle. The pathogenesis of fluke infections varies according to the number of metacercariae ingested and the phase of parasitic development in the liver. Clinically, these are difficult to detect, since the fluke burdens are usually low and anaemia is not apparent.
More often, fluke infections in cattle are subclinical, but they may cause reduced milk yields and changes in milk quality during the winter, with effects on fertility (Charlier et al, 2007). Economically, fluke infections in cattle are estimated to cost the UK agriculture industry about £300 million a year, with liver condemnations alone costing £3.2 million based on 2010 figures (source: Control of Worms Sustainably; COWS – www.cattleparasites.org.uk).
Fluke infections may be confirmed by demonstrating eggs in faeces, using either a McMaster technique with a floatation solution of high-specific density (zinc sulphate; ZnSO4) or a sedimentation method. Diagnosis can also be performed at the herd level to establish if the parasite is present on a farm as a possible cause of production loss.
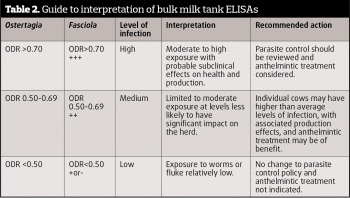
For dairy herds, bulk tank milk ELISAs can be routinely used to establish if a herd has been exposed to fluke. A positive correlation between Fasciola-specific bulk milk tank antibody levels and herd seroprevalence has been demonstrated and can be done routinely to determine levels of exposure (Table 2) and efficacy control programmes. The most recent survey of dairy farms across the UK in 2012 found 77.5% of farms in England, 88% in Wales and 73.4% in Scotland had been exposed to F hepatica, based on bulk milk samples (Howell et al, 2015).
Panel 1. Production effects of fluke infection in milking cattle
Fluke studies have shown that:
From a cut-off in ODR of 0.5 onwards, an average milk yield loss of 0.11kg/cow per day for each 0.1 unit increase in the bulk tank milk ELISA result
When the bulk tank milk ODR result exceeds 0.8:
– It is estimated that for 50% of the cows, the number of inseminations is increased by 75%
– The average intercalving interval of the herd is prolonged by 4-5
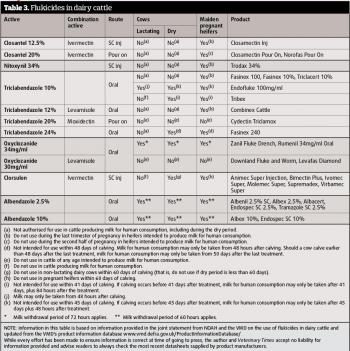
Fluke control strategies in youngstock and beef cattle rely heavily on flukicide treatments (Table 3) and should aim to reduce infection levels that minimise effects on cattle productivity. Control programmes should consider farm history and location, prevailing weather conditions and fluke forecasts.
Triclabendazole (TCBZ) is highly effective against all stages of immature fluke in cattle from more than 2 weeks when given orally (more than 8 weeks by pour-on). However, resistance to TCBZ appears to be an increasing problem and one requiring management on all livestock farms. Since acute fluke is rare in cattle, it is now suggested to only use TCBZ products in cattle when no other option is suitable.
Fluke control in adult dairy cows and pregnant heifers is less straightforward following European Commission regulation changes in July 2013 regarding the use of flukicides in cattle producing milk for human consumption. Few products are now authorised for sale on the UK market with permitted use in dairy cattle and dairy heifer replacements (see restrictions in Table 3).
Currently, the only products licensed for use in lactating dairy cattle to treat adult fluke are those containing albendazole (60-hour milk withhold) and oxyclozanide (72-hour milk withhold). Some products containing triclabendazole may be used at dry-off, with restrictions on when milk for human consumption may be taken (currently 44.5, 47, or 50 days) following treatment. Since product restrictions and withdrawal periods are liable to alter as maximum residue limits are determined for individual flukicidal products, it is important to continually check the most up-to-date data sheets.
Gastrointestinal worms, lungworm and liver fluke have been shown to reduce milk yield and growth and fertility rates in adult dairy cattle. As few worming or flukicide products are authorised for sale with permitted use in dairy cattle and dairy heifer replacements, it is important to monitor carefully parasite infections in dairy cattle, target anthelmintic treatments if considered necessary, and evaluate any production benefits.