6 Nov 2017
Hany Elsheikha looks at the groups of worms that infect cattle and discusses effective control and infection management.
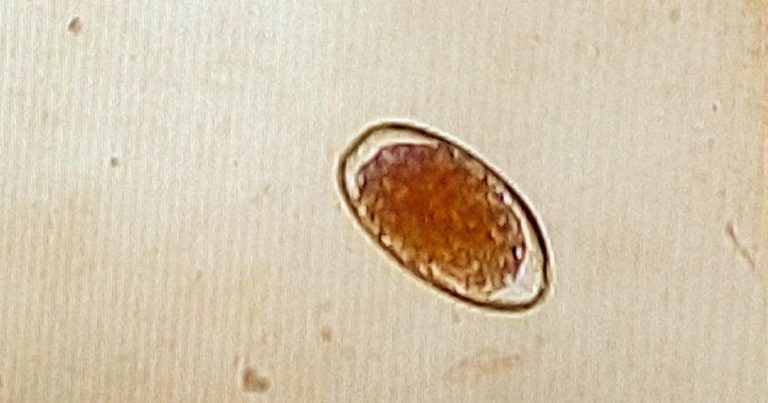
Figure 1. An egg (~85µm × 45µm) of abomasal nematode recovered from the faeces of cattle using the McMaster method.
Global food demand is expected to increase between 60% and 100% by 2050, as the human population is anticipated to reach around 9.7 billion. This significant population growth is anticipated to coincide with rising family incomes and consequent changes in dietary habits, such as eating more processed foods and meat, driving up global food demand. However, parasitic (nematode and trematode) infections can severely compromise the efficient production of safe and affordable dairy and meat products.
The cattle industry is also under threat from increasing levels of anthelmintic resistance. Livestock breeders will need to meet the demand for more protein by increasing productivity via breeding more animals, and by enhancing productivity through controlling parasitic diseases and adopting efficient methods to manage anthelmintic resistance. Therefore, meeting global food security without increasing pressure on livestock will be one of the major challenges for the industry over the next few decades, and shape livestock markets in ways we have not experienced before.
This article provides an overview of the groups of worms that infect cattle, discusses ways of effective worm control, and reviews the data on treatment and control of worm infection in cattle.
Gastrointestinal (GI) nematodes, such as Ostertagia ostertagi, are considered to be the most important nematode parasite group affecting cattle in the UK.
Traditionally, the timing of most anthelmintic control programmes have been designed to combat this abomasal nematode. Cattle can also be infected with other worm types, such as lungworms and liver flukes, which can adversely impact the health, fertility and productivity of dairy cattle (Morgan et al, 2013). Calves, particularly in their first grazing season, are more vulnerable to high parasite loads and succumb to clinical disease, leading to reduced growth rate and weight loss, which may persist into later life (Mason and McKay, 2006).
Changing climate conditions in recent years – for example, the increased pattern of warm wet summers and mild winters in the UK – may have favoured an increase in the number of snails, which act as intermediate host, facilitating the developmental life cycle of the rumen flukes and an increase in the incidence of rumen fluke infection in some geographic regions in the UK. Global climate change may also lead to a consequent increase in parasite infection, acute disease and death in young cattle. In the face of such worldwide changes, scientists have advocated the need to develop sustainable strategies for the effective control of ruminant worm infections (Morgan et al, 2013).
Worm infections are routinely treated with anthelmintics (wormers). Several anthelmintics exist that are effective against such infections (Elsheikha and Khan, 2011). The main chemical groups of anthelmintics are benzimidazoles, levamisole, imidazothiazoles and tetrahydropyrimidines, macrocyclic lactones, including avermectins and milbemycins, aminoacetonitrile derivatives (monepantel) and spiroindoles (derquantel).
Anthelmintics belonging to these chemical groups are active against the major species of GI roundworms and lungworms, with some also having activity against liver flukes. Macrocyclic lactones have activity against endoparasites and some ectoparasites, and are often referred to as endectocides. Other products, known as narrow spectrum drugs, are more specific in the parasites they kill, but are also active against certain ectoparasite species. Therefore, anthelmintic drugs should be chosen to target the species of worm (whether adult or juvenile form) highly likely to be involved in the infection, or that has already been identified using laboratory diagnostic methods, such as faecal worm egg count (Figure 1).
A number of deworming or flukicide products are authorised for sale in the UK, with permitted use in dairy cattle and dairy heifer replacements. Only one anthelmintic is known to be effective against rumen fluke, but this is not authorised in the UK as a veterinary medicine against this parasite. Given the limited pharmacological options to control these parasites in dairy cattle, improper use of anthelmintics could enhance the development of anthelmintic resistance (AR). Any increase in the prevalence of AR would render parasite control programmes unsustainable in the long term. Therefore, it is important to monitor parasite infection in dairy cattle and correctly diagnose the species of parasite infecting an animal so targeted treatment can be achieved in line with the Control of Worms Sustainably best practice guidelines.
Anthelmintics can be administered to cattle through a bolus, which falls into two types:
Boluses are designed for use primarily in first-year grazing animals at turnout, but can be used in cattle already at grass or in their second grazing season. Controlling parasites by selecting anthelmintics that have an effect on hypobiotic larvae allows the host to be cleared of a parasite burden before the worms cause clinical damage and reduces pasture contamination before the number of larvae in the environment reach levels that may cause disease in grazing cattle. For example, it is important to choose cattle anthelmintics effective against inhibited fourth stage O ostertagi larvae, which can cause type II ostertagiasis – resulting from the emergence of thousands of inhibited larvae from the stomach wall, leading to a serious disease known as winter scour.
A number of products are marketed as combined treatments for GI roundworms and liver flukes in cattle (for example, oxfendazole/oxyclozanide, levamisole/oxyclozanide, ivermectin/clorsulon and levamisole/triclabendazole). However, any decision to use these products needs to be carefully assessed.
Wherever possible, treatment should be given during the dry period. The milk withdrawal period should also be taken into account when it is necessary to treat dairy cattle with an anthelmintic. Many of the macrocyclic lactones (with the exception of eprinomectin) and the fluke products containing oxyclozanide or nitroxynil, should not be used in cows producing milk for human consumption. A licensed zero milk withdrawal, eprinomectin-based pour-on wormer is available to control worms in beef and dairy cattle, sheep and goats. This allows dairy animals to be treated at the optimal time without the risk of lost milk production.
Treatment options for GI nematodes include avermectins, milbemycins, benzimidazoles, probenzimidazoles, levamisole and morantel. Not all compounds are suitable for controlling hypobiotic larvae. Control methods differ widely according to climatic conditions, alternate grazing management options and the presence of AR. The major aim of GI nematode control is to maintain a safe grazing environment by reducing pasture contamination.

Control of liver fluke (Figure 2) infection in livestock relies heavily on the strategic use of a wide selection of flukicide drugs. Oxyclozanide, nitroxynil, clorsulon, triclabendazole and albendazole/ricobendazole, at increased dose rates, all have activity against adult liver flukes and are suitable for treating chronic fascioliasis. Triclabendazole is the drug of choice for treating cattle in late autumn and winter, a time when infection encompasses both adult and immature flukes, because it is also active against immature flukes.
Some products available to treat adult fluke are licensed for use in lactating dairy cattle, such as those containing albendazole (60-hour milk withhold) and oxyclozanide (72-hour milk withhold). Some products containing triclabendazole may be used at dry-off, with restrictions on when milk for human consumption may be taken following treatment. Cattle infected with rumen flukes can be treated with oxyclozanide. Control measures include avoidance or drainage of snail habitats, and anthelmintic treatments to prevent contamination of pastures with eggs.
Levamisole was one of the first drugs found to be effective against lungworms, and benzimidazoles and macrolides are more effective in the control of lungworm disease. Treating animals after the migration of larvae through the lymph nodes, and before the onset of clinical signs, is more effective in preventing disease and allowing the development of protective immunity.
An effective lungworm vaccine made from irradiated infective larvae primes the immune response against further exposure to infection. Two doses are given four weeks apart – at least two weeks before animals are turned out – to allow the development of a protective level of immunity. Incorporating vaccination into lungworm control programmes will help reduce the risk of any loss of performance as a result of potential worm burdens.
It is important to note some of the drugs used as wormers can lead to serious poisoning in humans. van der Veer et al (2015) reported an individual who developed severe lesions with discolouration and necrotised skin (particularly on the ears, nose and fingers) following exposure to levamisole-tainted cocaine, and another study reported a correlation between levamisole-adulterated cocaine and kidney damage in human patients (Collister et al, 2017). To avoid human poisoning, anthelmintics must not be stored with human medicines, must be kept out of children‘s reach and should never be stored in places where food is processed (such as kitchen and fridge) or people could mistake them for food.
Reports from a number of countries indicate AR in cattle GI nematodes is increasingly prevalent. AR can be defined as a decrease in the efficacy of a drug against a parasitic species that was previously susceptible, and develops due to multiple factors – especially underdosing, frequent treatments and low refugia (Elsheikha and Khan, 2011; Sutherland and Leathwick, 2011). The frequency of treatment is a potent source of selection pressure that occurs whenever a drug is often used. Resistant worms transmit their heritable traits to the next generation, thereby incrementally increasing the frequency of their genetic alleles in the general population. The genetic basis and modes of inheritance of resistance are complex, and vary widely among the various classes of drugs. Resistance to anthelmintics in GI nematodes of cattle is less prevalent than in sheep, but reports of infection with Haemonchus contortus, Haemonchus placei, Cooperia punctata, Cooperia oncophora, Cooperia spatulata and O ostertagi are increasingly common.
AR can threaten the sustainability of efficient farming practices considerably (McKellar and Jackson, 2004); therefore, strict measures should be taken to tackle this growing threat, with integrated management schemes and reduced dependence on using drugs.
Individuals who administer anthelmintics must read the product leaflet and summary of the product characteristics, and ensure the prescribed dose by weighing the cows, or using a weight band and well-maintained (calibrated and clean) dosing equipment.
Generally, anthelmintics can be given during the first two months of the grazing season to reduce the pasture contamination with worm eggs. However, it is important to base the treatment programme on evidence. A group faecal egg count at six to eight weeks after turnout, together with monitoring animals’ weight and condition, can give a good indication of whether early season treatment is necessary.
The rate at which resistance to a certain anthelmintic drug develops is unknown and, with limited alternative options to worm control, makes routine monitoring of the efficacy of anthelmintic drugs at farm level a necessary measure for effective parasite management. AR in cattle nematodes can be detected using a faecal egg count reduction test. Although the test is not sensitive enough to detect AR in the early phase of development, it can detect resistance before clinical treatment fails.
It is important to take a holistic approach towards controlling worm infections. While the strategic use of antiparasitic drugs is valuable in managing adverse impacts of parasitism on animals, grazing management tools, such as provision of clean pastures, alternate grazing by other animal species, alternate grazing by immunologically resistant hosts of the same species and monitoring of parasite transmission, can also influence the effectiveness of drug regimes. Sound pasture management reduces the need for anthelmintics, minimises reinfection by preventing recontamination of spring pastures, and prevents the midsummer build-up of infective nematode eggs or larvae on pasture (Elsheikha and Khan, 2011).
Refugia (those in refuge) refers to the subpopulation of parasites not exposed to the anthelmintic at the time of treatment. This includes larval stages in the environment, parasites of some individual animals left untreated, and parasitic stages within the animal not exposed to the treatment due to physiologic or pharmacokinetic factors. The subpopulation in refugia represents a reservoir of unselected genes and provides a source of drug-susceptible worms to mate with drug-resistant worms. Any increase in the refugia will lead to a reduction in the rate of resistance development and vice-versa.
Food security – maintaining the availability of food in the face of increasing global demand – is one of the greatest and most important challenges faced by humans today. Farm animal vets, field experts, clinical parasitologists and SQPs can all play a key role in maintaining efficient livestock production by preventing and treating parasitic diseases. Maintaining the effectiveness of existing anthelmintic drugs is essential for ensuring the overall success of livestock worm control.
Treatment should be supported by frequent monitoring of worm burdens and the adoption of parasite management practices that delay the development of resistance. Farmers must be well-educated on the proper use of anthelmintics and ensure they are able to make informed decisions – following evidence-based guidelines on the effective use of cattle wormers (http://bit.ly/2irEUxl). Worm management in cattle should rely on an integrated approach, which combines anthelmintic treatment and good grazing management.
The author declares no commercial or financial relationships that could be construed as a potential conflict of interest. References to drugs do not imply their endorsement by the author or publisher. It is important to read the most up-to-date product data sheets, as some restrictions on products, including withdrawal periods, are prone to change as maximum residue limits change for individual products.