21 Mar 2016
Karen Walsh discusses anaesthetic management options for the more common endocrinopathies seen in general practice, as well as considerations for these cases.

Figure 2. Mature diabetic cataracts.
As the number of geriatric patients in the veterinary population increases, the number of patients with concurrent diseases, including endocrinopathies, will be more prevalent.
These patients may live with these conditions for many years and may require anaesthesia for unrelated issues in this time.
Understanding the pathophysiology of the endocrinopathy, and the clinical syndromes associated with it, will enable the clinician to pre-empt problems that may occur.
Where possible, treatment for the endocrinopathy should be initiated before anaesthesia and surgery. This will improve function and, hopefully, decrease the likelihood of morbidity.
The physiological effects of hypothyroidism are:
Of particular interest to the anaesthetist is the possible impaired cardiac contractility.
In general, these patients may require lower doses of sedatives and anaesthetic agents, and should be closely monitored from premedication to recovery. They may also be more likely to become hypothermic and take longer to reach normal temperature, because of the suppressed level of metabolism.
Laryngeal paralysis has been associated with hypothyroidism. An increased inspiratory effort, often with an inspiratory stridor, may indicate its presence. The clinician should be ready for a difficult endotracheal intubation and may wish to evaluate laryngeal function at the time of anaesthetic induction.
If laryngeal paralysis is suspected, but not being surgically corrected, the patient should be closely monitored during recovery for upper airway obstruction.
Where possible, patients should be actively warmed from the time of premedication and be closely monitored during recovery until they return to normal body temperature.
Treatment should be initiated before anaesthesia and surgery. This will improve function and, hopefully, decrease the likelihood of morbidity.
Hyperthyroidism is most common in cats and some owners may elect for a surgical treatment. However, it is important to stabilise thyroid function before general anaesthesia, as cardiovascular function may be severely compromised.
The physiological effects of hyperthyroidism are:
Hypertrophic cardiomyopathy is the most common cardiac disease that occurs secondary to hyperthyroidism in cats. They may exhibit gallop rhythm, tachycardia and, sometimes, pleural effusion. Hypertrophic cardiomyopathy is often reversible when treatment is started.
When the patient has been treated for hyperthyroidism, it may show signs of renal insufficiency (due to decreased glomerular filtration rate) and renal perfusion, so renal parameters and urine-specific gravity should be evaluated after stabilisation of the thyroid disease.
Patients may also experience hypertension, which should be evaluated pre and post-treatment for thyroid disease. Treatment should be initiated for hypertension that does not resolve with stabilisation of thyroid disease prior to general anaesthesia.
A few cases may require anaesthetising or sedating with uncontrolled hyperthyroidism; the owner should be informed of the risks involved, which may include worsening of cardiac function and death.
Assuming the patient has been adequately stabilised and renal parameters are within normal limits, the anaesthetic plan should be tailored to the individual patient and procedure (Table 1). General considerations to be aware of include:
Many patients with unstable hyperthyroidism may be difficult to handle, which can make performing diagnostic tests a challenge. The considerations taken for stable hyperthyroid patients remain valid and the aim of sedation or anaesthesia is to maintain cardiovascular function to as close to normal as possible.
An echocardiogram to assess cardiac function may be an advantage, if it is possible, to allow appropriate anaesthetic planning.
If a hypertrophic obstructive cardiomyopathy exists, evidence suggests using medetomidine to slow the heart rate and allow a more effective contraction (Lamont et al, 2002).
However, without echocardiography, it is wise to avoid the use of alpha-2 agonists in patients experiencing cardiac symptoms due to hyperthyroidism. The main aim is to keep heart rate about similar to the resting heart rate to maintain cardiac output.
The “stress response” is the body’s way of dealing with noxious stimuli; this system activates during anaesthesia and surgery.
An impaired endocrine system may influence the stress response to anaesthesia and surgery, changing the risks associated with both of these.
In some cases, it may be appropriate to initiate the stress response by administering steroids at the time of anaesthesia and surgery, allowing a physiological response to noxious stimuli (discussed more in the hypoadrenocorticism section).
Hypoadrenocorticism is caused by a deficiency of aldosterone and, to a certain extent, glucocorticoids. The physiological effects are:
Stabilising these patients prior to general anaesthesia is essential, especially with respect to potassium levels and the possible secondary cardiac arrhythmias. When preparing to anaesthetise a patient being treated for hypoadrenocorticism, take a blood sample to check electrolytes are within normal limits.
Assuming all is normal, the patient can be admitted as per routine on the day of the surgery and any medication should be continued as usual. Anaesthesia premedication and induction can be carried out as normal.
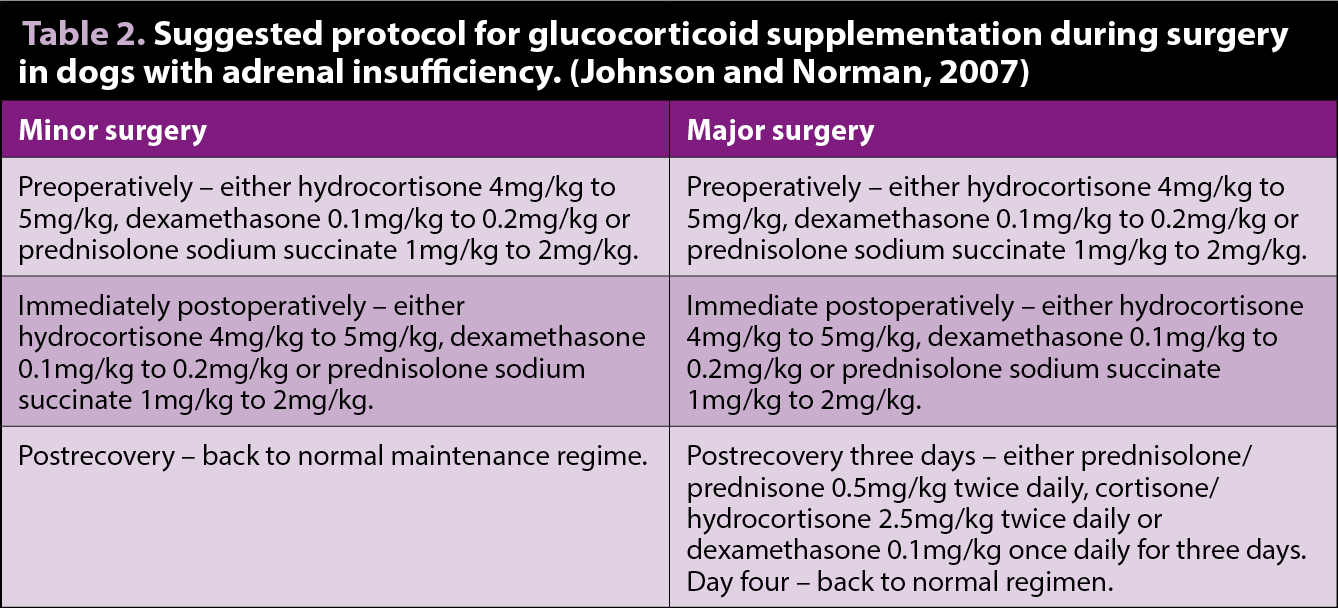
One factor to take into consideration is the patient’s ability to mount an effective stress response in the face of anaesthesia and surgery. The author will often supplement the patient with exogenous steroids. The amount administered depends on the perceived severity of the stress – for example, major or minor surgical procedures (Table 2).

Cushing’s disease is characterised by an overproduction of glucocorticoids. This excess steroid can lead to muscle weakness, redistribution of fat to the cranial abdomen, generalised neuropathies and coagulation abnormalities (Figure 1). Problems related to this disease, their impact on anaesthesia and their possible solutions are listed in Table 3.
These patients are at higher risk of pulmonary thromboembolism due to the altered coagulation function. Early mobilisation after surgery may help reduce clot development, so adequate analgesia is essential. However, it is probably prudent to avoid NSAIDs as they may be synergistic with the high levels of circulating steroids present in these patients.
Maintaining adequate perfusion is essential during anaesthesia, so monitoring blood pressure is desirable. Low doses of acepromazine (0.005mg/kg to 0.01mg/kg IV or IM) may allow some vasodilation without causing hypotension. This, combined with an opioid, may allow stress-free handling.
Medetomidine should be avoided in hypertensive patients, as it will produce an initial hypertension. It may be helpful at anaesthesia induction to administer supplemental oxygen for five minutes beforehand, in an effort to reduce the incidence of hypoxaemia.
Although unlikely to perform surgery to remove a pancreatic mass in general practice, clinicians may have to sedate or anaesthetise a patient with a suspected insulinoma when making a diagnosis.

Insulinomas produce excess insulin, thereby making the patient hypoglycaemic. Hypoglycaemia may be controlled by small frequent meals, sometimes with additional steroid therapy. Preoperative preparation should, therefore, be carefully considered.
Minimise preoperative fasting in an effort to reduce the likelihood of hypoglycaemia. If it does occur, bolus administration of glucose should be avoided as this can lead to increased insulin release and, therefore, a rebound hypoglycaemia. Instead, a slow increase in glucose should be initiated using 2.5% to 5% glucose solutions. The author often supplements Hartmann’s solution with glucose to deliver a balanced electrolyte solution (see diabetes section).
Incorporating medetomidine into the anaesthetic protocol may have some advantages, as it is an insulin antagonist and will help increase glucose levels during sedation and anaesthesia (Guedes and Rude, 2013). Monitoring blood glucose approximately every 60 minutes to 90 minutes during the perioperative period will help pre-empt any hypoglycaemic episodes.
If anaesthesia for pancreatectomy is performed, blood glucose should be measured regularly postoperatively.
Hyperglycaemia and hypoglycaemia may occur after insulinoma removal. Reports exist of propofol increasing the likelihood of pancreatitis. The mechanism of this is not entirely understood, but may be related to the large lipid load administered.
Pancreatitis can also result from surgical handling of the pancreas, disruption of the blood supply and hypotension during surgery.
Unless it is a life-threatening emergency, anaesthesia should only be performed on patients that have had their diabetes stabilised. Patients that have not been stabilised may be ketoacidotic with severe metabolic dysfunction (Table 4).
Diabetes effects of particular importance to anaesthesia are:
Hypoglycaemia can cause irritability, seizures, bradycardia, hypotension and respiratory failure.
All can be masked by general anaesthesia and sedation; therefore, blood glucose should be monitored during the procedure.
Diabetic patients may also be more susceptible to the effects of hypoglycaemia at higher glucose levels than a healthy patient, though the exact levels have not been established in canine and feline patients.
It is useful to have a checklist or “crib sheet” to follow when admitting diabetic patients. Questions to include are:
Even if a patient is stabilised with regard to its diabetes, it is very common anaesthesia and surgery will lead to a short period of destabilisation. Returning the patient to its normal regime as quickly as possible is the main aim.
There are no hard rules for patients receiving once-daily insulin, but it is more practical and logical to perform surgery in the morning to allow minimal disruption of the regime. Ask the owner to feed the pet as normal the night before, but withhold food and insulin in the morning. Panel 1 is the protocol used in the author’s clinic.
Few studies exist into the optimal insulin regime around the time of anaesthesia. One study investigated the use of full-dose insulin, compared to a quarter of the usual dose, and found both regimes resulted in unpredictable blood glucose levels. The full dose of insulin only gave marginal benefits over the reduced insulin dose (Kronen et al, 2001).
For morning surgery, use the same regime as for patients receiving once-daily insulin, until the end of surgery. When the surgery is finished, the patient should be encouraged to eat when safe. Blood glucose should be measured if the patient does not eat, particularly when peak insulin activity is expected. The aim is to try to return to a normal regime as soon as possible, to be able to administer the evening insulin dose.
Preoperative fasting can be limited to six hours for anaesthesia for afternoon surgery (Savvas et al, 2009). It is important to try to time the anaesthesia so it is six hours after the last meal – either move the morning feeding and insulin forward a little, or delay anaesthesia.
Ask owners to feed as normal and give the usual dose of insulin in the morning. There is no need to perform a blood glucose if the patient has eaten well that morning, unless there has been a recent history of hypoglycaemic episodes.
Perform blood glucose at the time of induction of anaesthesia and every 30 minutes to 60 minutes thereafter.
If blood glucose has been within normal limits during surgery, feed when the usual schedule dictates and carry on insulin therapy as usual.
Considerations for anaesthesia and surgery include:

In ketoacidotic patients, the anaesthetic risk is high; therefore, stabilisation prior to anaesthesia is vital.
In the rare cases where surgical intervention or anaesthesia cannot be delayed, consider the following points:
Cardiovascular collapse can occur secondary to severe dehydration, due to diuresis and fluid deprivation, and/or myocardial depression, due to severe metabolic acidosis. Rapid correction of diabetic ketoacidosis can result in cerebral oedema.
Fluid therapy should be tailored to any blood results and the clinical condition of the patient.
Hyperkalaemia correction before anaesthesia is important and a balanced electrolyte solution can help this. A 2010 study showed sodium chloride or Hartmann’s solution could be used to rehydrate cats with hyperkalaemia due to urethral obstruction, but Hartmann’s was more effective at treating acidosis (Cunha et al, 2010).
This could be extrapolated to indicate using Hartmann’s solution in diabetic ketoacidosis may be more sensible as it will help to alleviate the acidosis and electrolyte disturbances. In addition, appropriate insulin therapy should be started and monitored.
Parameters that can be objectively measured are:
Subjective assessments of cardiovascular status are:
If the patient is not adequately stabilised, the metabolic acidosis present will result in an increased effect of anaesthetic agents. Induction agents should be given slowly to effect and depth of anaesthesia should be monitored closely.
The patient will also be more susceptible to the effects of hypoxaemia, so pre-oxygenation may be advisable and the airway should be rapidly secured.
Where possible, end-tidal carbon dioxide should be monitored and, if greater than 45mmHg, intermittent positive pressure ventilation should be started to avoid worsening of the pre-existing acidosis.