13 Dec 2010
Simon Tappin discusses the diagnostic value of bronchoscopy in dogs and cats, and examines techniques.

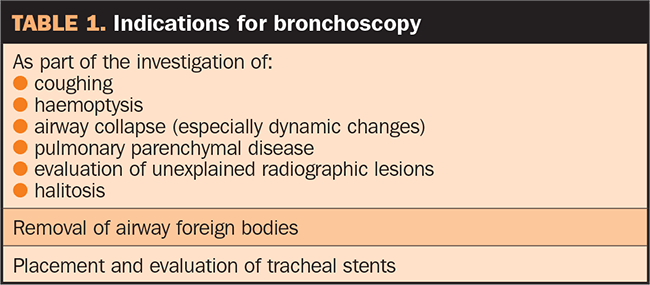
Figure 1. A 4mm human paediatric bronchoscope, suitable for bronchoscopy in small to medium dogs.
Bronchoscopy – or, more correctly, tracheobronchoscopy – allows evaluation of the larynx, trachea and bronchial tree.
The level to which they can be examined depends on the diameter and length of the endoscope used. However, evaluation to the level of the tertiary bronchi is possible in most cases.
As with other forms of endoscopy, although gross evaluation may reveal a definitive diagnosis (such as the presence of an airway foreign body), it is most commonly performed at the end of a diagnostic pathway.
As always, this should start with a full history and thorough clinical examination, and may include haematology, biochemistry, faecal parasitology and thoracic radiographs. If imaging is performed it is often done under the same anaesthetic as bronchoscopy (as inflated views may be more diagnostic), but it should always be performed prior to the endoscopic procedure.
Bronchoscopy is useful in many patients (Table 1), but is most commonly used when assessing chronic coughing, respiratory distress and haemoptysis. Samples can also be collected and submitted for cytology and culture.
Flexible fibreoptic or video bronchoscopes are preferred for bronchoscopy, as they allow a thorough evaluation of the respiratory tract (Figure 1).
Paediatric bronchoscopes with a diameter of 3mm to 4mm are used in cats and small dogs, but their working length is usually short (50cm to 60cm), which can be prohibitive in larger patients.
In this case, adult bronchoscopes (diameter 5mm to 6mm) or fine paediatric gastroscopes may be used as they have a longer working length (100mm to 120cm).
Bronchoscopes usually only have two-way deflection (up and down), but rotation can be achieved by twisting the hand piece and, therefore, the insertion tube gently along its long axis (Figure 2).
Rigid fibreoptic endoscopes can also be used and are valuable for evaluating the larynx and trachea, although evaluation of the lower airways is difficult. Cytological samples cannot be directed with a rigid endoscope. Hollow rigid endoscopes can be useful to allow removal of tracheal foreign bodies.
Bronchoscopy is always performed under general anaesthesia to control reflex coughing and gagging on passage of the endoscope.
Premedication with an opioid and low-dose acepromazine is used in most cases. Terbutaline, a bronchodilator, is useful to reduce bronchospasm and improve oxygenation. This is especially helpful in cats and is usually provided intra-muscularly at the time of induction. General anaesthesia is most easily maintained by total intravenous anaesthesia (TIVA), with propofol being the agent of choice in most cases. This allows endoscopy without the risk of leakage of anaesthetic gases.
In large dogs, the endotracheal tube may have a large enough diameter to allow passage of the endoscope without complete occlusion of the tube (at least 25 per cent of the diameter of the tube should remain with the endoscope in place). If this is the case, a T-adapter may be used to allow oxygen delivery (anaesthesia is usually still maintained via TIVA, as there is still a risk of anaesthetic gases escaping; Figure 3).
Unfortunately, most cats and small dogs have airways too narrow to allow the endoscope to pass through the endotracheal tube. In these cases, the endoscope is advanced directly into the airway to allow evaluation. To facilitate this, the patient is anaesthetised and an endotracheal tube placed, initially to allow stabilisation of the patient and, if needed, inflated thoracic radiographs to be taken.
Once ready, the endotracheal tube is removed and bronchoscopy is performed. Local anaesthetic can help reduce laryngospasm as the endoscope is passed. Once the bronchoscopy is finished, the endotracheal tube is replaced to allow the patient to recover.
Endoscopy without an endotracheal tube does not allow assisted ventilation, so careful consideration should be given to oxygen supplementation. This can be provided through the endoscope wash channel, via a urinary catheter placed in the airway alongside the endoscope or via flow by oxygen. Oxygen flow rates of one to three litres a minute can be used safely in most cases. Care should be taken not to over-inflate the small airways and the patient should be carefully monitored to ensure adequate oxygenation; a pulse oximeter is very useful in this respect.
Tracheobronchoscopy is best performed in sternal recumbency, with the head elevated on a sandbag or foam pad to allow easy passage of the endoscope. A gag should be placed to protect the endoscope.
A laryngoscope is helpful to guide the passage of the endoscope through the larynx into the cervical trachea, which should be near circular in normal patients. The tracheal cartilages are seen as C-shaped rings under the mucosal surface and are connected by the dorsal membrane (Figure 4). This thin strip of muscle helps to orient the picture and should not deviate into the airway.
The tracheal mucosa should appear smooth, with a light pink appearance. Submucosal vessels are normally visible, but become more prominent in inflammatory disease (Figure 5).
Inflammatory disease can also lead to the airway having a hyperaemic, oedematous appearance. Bleeding is possible on passage of the endoscope and polypoid lesions are occasionally seen in chronic inflammatory conditions.
Oslerus osleri infection leads to parasitic nodule formation in the distal trachea and larger bronchi. A small amount of airway secretion is normal in cats and dogs. Excessive mucus secretion is usually associated with chronic inflammatory processes, such as chronic bronchitis and eosinophilic bronchopneumopathy. Airway haemorrhage can also be seen in association with trauma, foreign bodies and Angiostrongylus infection (Figure 6).
In small dog breeds tracheal collapse is relatively common and changes in airway diameter should be assessed during each phase of respiration, as collapse is often a dynamic process (Figures 7 and 8). If medical management fails to control signs of tracheal collapse, then endotracheal stent placement can be considered. Tracheoscopy is used to help stent deployment and to assess placement (Figure 9).
Once the cervical trachea has been examined, the endoscope is gradually advanced, through the intrathoracic trachea to the tracheal bifurcation or carina. The endoscope should be centred in the middle of the airway to avoid trauma to the mucosal surface. The tracheal bifurcation is seen as a sharp division between the left and right mainstem bronchi (Figure 10). The right mainstem bronchus is usually straight ahead of the endoscope, with the left mainstem bronchi requiring some deflection to the right to allow entry.
For this reason, airway foreign bodies are more commonly seen in the right mainstem bronchi (Figure 11). Segmental collapse can occur in the mainstem bronchi (alone or in association with tracheal collapse) and the left atrial enlargement can cause left mainstem bronchial compression (Figure 12).
The bronchial tree should be systematically and fully evaluated, allowing visualisation of each lobar bronchus and as many segmental divisions as possible. Each lung has a cranial and caudal lobe, with the right side having both a middle and accessory lobe (Figures 13 and 14).
Each segmental airway should be evaluated and changes in shape, size and mucosal appearance noted. The normal lower airways should have crisp divisions between the airways and have a pale pink mucosal appearance (Figure 15).
Once the airways have been fully examined, samples should be obtained for cytology and microbiology, as gross changes are not usually pathognomonic for specific disease.
Sampling is best achieved via bronchoalveolar lavage (BAL), allowing material to be collected from the lower airways, which avoids potential contamination from the upper respiratory tract. Samples are obtained by wedging the endoscope into a terminal bronchus and instilling saline. This is best done via a wash tube inserted through the working channel of the endoscope (Figure 16), but can be performed using a wash trap directly through the wash channel (Figure 17).Aliquots of sterile saline (0.9 per cent sodium chloride) are instilled to allow collection of representative samples. Typically 1.0ml/kg saline is used, with aliquots of 3ml to 5ml used in dogs and 5ml to 10ml in larger dogs. Saline is absorbed readily from the lower airways so, although care should be taken, patients cope very well with the fluid volume instilled. Typically, only 25 per cent to 75 per cent of wash fluid is retrieved and should be stored into ethylenediaminetetraacetic acid (EDTA) tubes for cytology and plain tubes for culture.
If the wash channel was used for sample collection, culture results should be interpreted with caution, and saline culture flushed through the wash channel prior to BAL collection may help exclude contamination. Culture results must always be interpreted in the light of the cytology results obtained.
Once positioned in a terminal bronchus, saline is instilled and an assistant applies coupage to the dog’s chest. The fluid is left for a few seconds and then suctioned via the wash tube or endoscope channel. Moving the endoscope gently backwards and forwards by a few millimetres during suction may aid sample collection (Figure 18).
Flushing is repeated until good samples are obtained. These should have a frothy appearance as a result of surfactant being present (Figure 19). BAL samples should be obtained from at least two sites (usually the left and right sides) as well as any focally abnormal areas. Brush cytology and aspirates taken via endoscopic injection needles can be helpful in investigating focal abnormalities and airway masses.
Biopsies can also be taken, with forceps inserted through the working channel. These are helpful both for investigating masses and collecting samples for electron microscopy to evaluate ciliary function.
Post-bronchoscopy, the patient is usually intubated and maintained on 100 per cent oxygen until stable, and then recovered from anaesthesia. Patients need to be monitored closely on recovery and supplemental oxygen provided as needed. They are generally hospitalised for 12 to 24 hours after the procedure and monitored closely for complications.
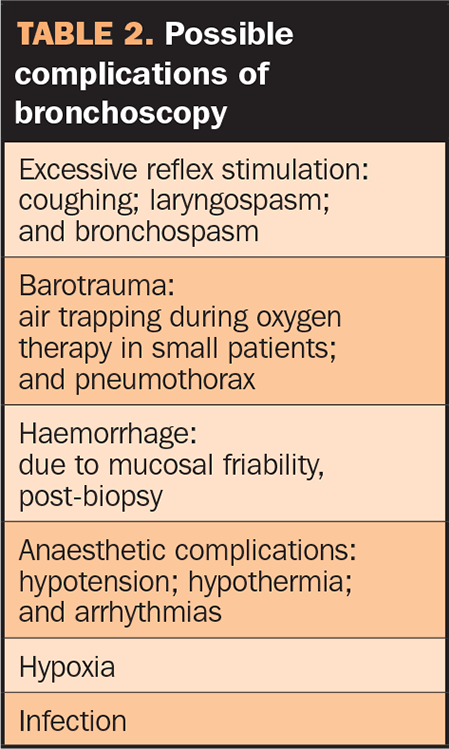
Bronchoscopy is generally a safe procedure, however, a number of complications are possible (Table 2). Perhaps the most common is bronchospasm seen post-bronchoscopy or BAL in cats, which can lead to severe respiratory distress immediately after the procedure or on recovery. Pre-treatment with terbutaline may reduce its incidence. Oxygen supplementation and intravenous or inhaled steroids may be needed to control airway constriction post-procedure.