9 Dec 2019
Maria-Christine Fischer in the first of a two-part article, describes lens characteristics, cataract causes and untreated case complications.
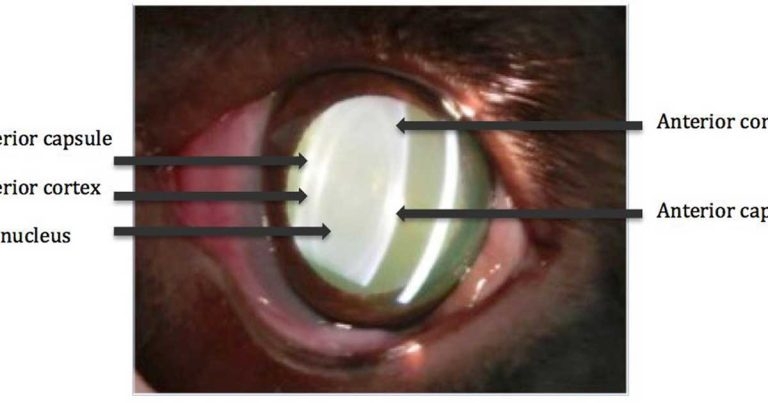
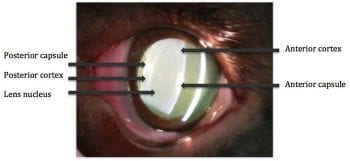
Figure 1. A slit lamp image of a lens showing the capsule, cortex and nucleus.
Cataracts are the leading cause of vision loss in dogs. Due to great progress in cataract surgery in the past few decades, lens opacities can now be removed surgically, with reported success rates of up to 80% to 90%1,2.
This article describes anatomical and physiological characteristics of the lens, and subsequently discusses the common causes and complications of untreated cataracts. Part two will focus on essential steps of the examination and treatment.
The lens is a transparent, biconvex and avascular tissue that refracts light. Via accommodation, a dynamic change in the refractive power, near and distant objects can be focused on the retina. In comparison to people, the accommodative power of dogs is much smaller and canine lenses don’t change shape, but move anteriorly in the eye during accommodation3.
The transparency of the lens is a result of precise and regular arrangement of lens fibres, and is maintained by metabolic processes. Anaerobic glycolysis provides most of the energy requirements of the normal lens. Disturbances in the aqueous humour composition, such as elevated glucose levels in diabetic patients, can affect the lens metabolism and transparency.
The lens is composed largely of protein (35%), water (65%) and electrolytes. It can be divided into three layers: the lens capsule, lens cortex and lens nucleus. As new lens fibres are formed throughout life in the cortex, older fibres become more tightly packed, and the lens nucleus becomes denser and harder. This results in age-related nuclear sclerosis and loss of lens elasticity. People, therefore, experience farsightedness (presbyopia) with increasing age4.
Nuclear sclerosis is the most important differential diagnosis for cataracts and can be difficult to distinguish without an ophthalmic examination. In dogs older than six to eight years of age, nuclear sclerosis becomes visible as a bluish-grey haze and the nucleus might appear as a “lens in the lens” (often visible as a “halo” on distant direct ophthalmoscopy).
Fundoscopy is still possible in nuclear sclerosis, but not in case of a nuclear cataract. In most animals, nuclear sclerosis hardly affects vision and cataract surgery is not required5.
As cataracts have a highly variable nature and appearance, different methods of classification are commonly used in combination. Cataracts can be classified according to their state of progression, location (for example, nuclear, cortical, equatorial, anterior or posterior subcapsular), age of onset (congenital, juvenile, senile) and aetiology.
Inheritance is probably the most common cause of cataracts6, which is an important consideration if a dog with hereditary cataracts is to be used for breeding7. Hereditary cataracts are not associated with systemic or other ocular diseases8. These lens opacities develop most commonly in the first years of life or can occasionally be congenital8-12. Table 1 lists hereditary cataracts for different breeds with typical location, age of onset and mode of inheritance. So far, approximately 20 dog breeds are proven to have a primary hereditary cataract6.
Congenital cataracts are present at birth. They may be inherited (Table 1), the result of in utero infection or toxicity, or appear together with/secondary to other ocular developmental abnormalities7. The latter include persistent pupillary membranes and a persistent hyperplastic tunica vasculosa lentis/persistent hyperplastic primary vitreous. Microphthalmia and multiple ocular anomalies can also be associated with cataract formation6.
Some congenital lens opacities are static, whereas others progress7. In the case of severe visual impairment, cataract surgery should be considered because lack of visual input can result in irreversible functional visual deficits7. However, following cataract surgery in a young animal, the absence of the lens may influence the ocular growth and result in further complication.
Some ophthalmologists recommend postponing surgery until the patient is fully grown. Spontaneous resorption of cataracts may be seen in some patients13.
Diabetes mellitus is the most common cause of metabolic cataracts in the dog. Despite control of hyperglycaemia, a study of 200 dogs showed 50% of diabetic patients developed a cataract within 170 days of diagnosis and 80% after 470 days14.
Hyperglycaemia results in an accumulation of glucose in the lens and overwhelms the normal metabolic pathway within the lens fibres (anaerobic glycolysis).
The excess glucose is instead “shunted” to an alternative metabolic pathway, where the enzyme aldose reductase converts glucose to sorbitol, which accumulates in the lens. This results in hyperosmolarity of the lens and swelling of the lens fibres due to fluid ingress. A complete cataract and vision loss can develop within days.
Typically the lens is swollen, displaying an intumescent cataract with characteristic “waterclefts” along the lens suture lines. The swelling of the lens may cause rupture of the lens capsule resulting in severe (phacoclastic) lens induced uveitis (LIU). The lens capsule rupture may be difficult to see on an ophthalmic examination as it frequently occurs at the equator or the posterior lens capsule. Ocular ultrasound can further help assess lens capsule rupture.
As secondary glaucoma and loss of retinal function are not uncommon in the presence of LIU, early surgical intervention prior to development of complications is recommended15 even if diabetes mellitus is not well controlled.
During toxicological screening of new pharmacological substances, several cataratogenic drugs have been found in laboratory dogs. However, most of these agents were used chronically in high non-therapeutic doses, and these are not in common veterinary usage.
A clinically relevant type of toxin-induced cataract is associated with progressive retinal atrophy (PRA) and other forms of retinal degeneration6. It has been suggested that degenerated photoreceptor cells in the retina release dialdehydes, which diffuse through the vitreous and may by toxic for lens cells16.
The initial lens opacity seen in dogs with PRA, frequently at the posterior pole of the lens, may support this theory6,16. However, this suggested pathogenesis has not been proven – it is also possible that two separate hereditary diseases exist in these patients, that is, they have PRA and a predisposition to develop cataracts6,7.
Patients undergoing radiotherapy to treat neoplasms in the head region can develop lens opacities if the eye is in the radiation field.
A traumatic cataract can result from contusion and/or perforation of the lens. A severe blunt trauma can damage lens epithelial cells and subcapsular lens fibres causing variable degrees of cortical lens opacity. Contusion cataracts may also manifest along the lens suture lines6 and are displaced into deeper layers as new lens fibres are formed23.
Cases with perforating lens trauma should be referred as an emergency and treated aggressively. Small lens capsule rents (less than 1.5mm) may seal spontaneously, but for larger lens lacerations with extrusion of lens material, lens removal has been recommended24.
A more recent study even reported that lens laceration up to 4mm may seal spontaneously and can be managed medically27,28. Long-term monitoring for cataract progression and complications is required, and long-term intensive treatment should be expected with perforating lens injuries; however, some cases may remain refractory to treatment.
Severe or chronic uveitis6,29, chronic glaucoma30,31, primary lens instabilities and retinal degeneration6 can result in cataract formation via changes to the lens metabolism32.
Complications occur at any stage of development, but more frequently the further the cataract has progressed. The risk of complications is, therefore, highest for hypermature and lowest for incipient cataracts35. The most frequent ocular complication associated with cataracts is LIU35. The pathogenesis of LIU is complex and not fully understood36.
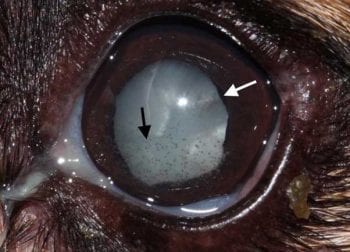
It is assumed a T-cell tolerance to the lens is normally maintained by a small amount of circulating lens protein37,38. Increased amounts of lens protein – especially with hypermature cataracts38,39,40, lens capsule rupture15 or extracapsular lens extraction41-43 – most likely overwhelm the T-cell mediated tolerance to lens proteins, resulting in initiation of an immune-mediated inflammation. LIU can occur at any stage of cataract progression44,45; the prevalence was as high as 71% in patients examined prior to undergoing cataract surgery46.
Two types of LIU are described. Phacolytic uveitis occurs when degraded lens protein leaks through the intact lens capsule47,48,39,40. It is the most common form of LIU and should be suspected in all eyes with cataracts that show signs of redness and inflammation49. Aggressive anti-inflammatory treatment is indicated initially and most patients require some form of life-long therapy.
Phacoclastic uveitis is present in eyes with traumatic or spontaneous (for example, with rapidly developing, intumescent diabetic cataracts) lens capsule rupture when a massive amount of lens protein is released into the eye39. The risk for loss of vision is high as this condition responds poorly to anti-inflammatory treatment. Therefore, if phacoclastic LIU is present, rapid surgical removal of the lens is indicated for most cases38.
Early studies on canine cataract surgery reported a reduced success rate of cataract surgery for eyes with LIU46. This may be less significant with improved surgical techniques50. However a pre-operative anti-inflammatory therapy with topical corticosteroids or NSAIDs and systemic NSAIDs is recommended39,40,46.
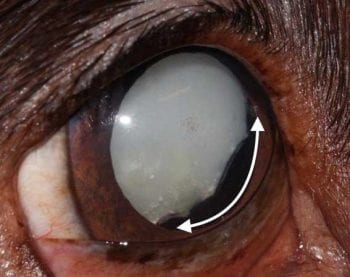
Clinically, LIU presents with one or more of the following symptoms; reduced intraocular pressures (normal reference range 10mmHg to 25mmHg), miosis, aqueous humour flare or cells, swelling of the iris, posterior synechia, conjunctival hyperaemia, congested episcleral vessles, photophobia and blepharospasm.
Hypopyon, fibrin and hyphaema can also occur in rare cases. Eyes with a subtle, chronic LIU may have a hyperpigmented iris, ectropion iridis, anterior or posterior synechia (adhesions between the iris and cornea and/or iris and lens), pigment dispersion on the anterior lens capsule, and medical mydriasis may be slow and incomplete38,40,43,51.
The glaucoma may be the result of various mechanisms: accumulation of inflammatory debris in the drainage angle; forward displacement of the iris by a swollen (intumescent) lens – for example, in diabetic cataracts and subsequent narrowing of the iridocorneal angle; damage to – and swelling of – the trabecular meshwork as a result of the inflammatory process; formation of anterior or posterior synechia; or formation of inflammatory membranes (periridal fibrovascular membranes) that occlude the pupil or the ciliary cleft33,39.
Further complications of cataracts include vitreal degeneration, retinal detachment, and lens luxation or sub-luxation. These occur more frequently with long-standing, hypermature cataracts.