27 Nov 2017
Albane Fauron and Karen Perry, in the final instalment of a three-part article, discuss management options for cranial cruciate ligament ruptures.
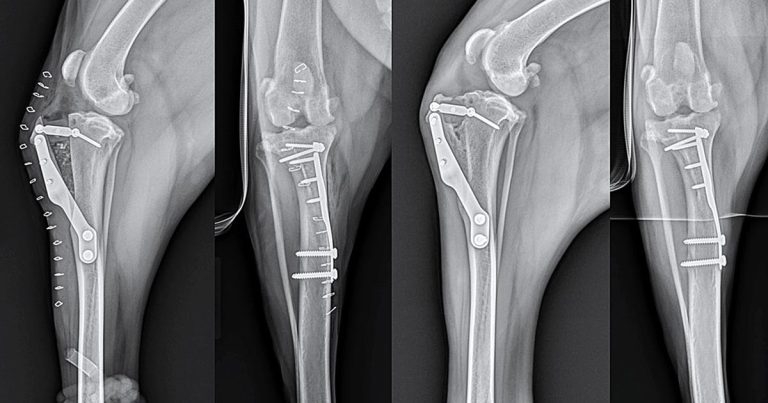
Figure 4. Mediolateral and caudocranial views taken immediately postoperatively (left) and eight weeks postoperatively (right) following routine tibial tuberosity advancement for stabilisation of a cranial cruciate deficient stifle in a five-year-old Labrador retriever.
While conservative management of cranial cruciate ligament rupture may result in an acceptable outcome, especially in dogs less than 15kg, surgical management remains the gold standard. A variety of techniques have been designed to stabilise the cranial cruciate deficient stifle. This article – further to parts one (VT47.24) and two (VT47.31) – focuses on presenting the ones most commonly performed.
Surgical management should always include full joint exploration and treatment of any meniscal injury. In the absence of meniscal injury at time of surgery, meniscal release may be performed, but remains controversial. Ultimately, the choice of stifle stabilisation technique depends on the surgeon’s experience and preference, as many techniques result in good to excellent outcomes and return to function.
Only a few peer-reviewed reports exist on the outcome associated with conservative management of canine cranial cruciate ligament (CrCL) rupture.
Vasseur (1984) retrospectively evaluated the outcome of 85 dogs diagnosed with CrCL rupture that were treated with exercise restriction, weight loss when indicated and analgesia when needed, and identified bodyweight as an important factor in determining the eventual outcome for these dogs.
In this report, 85.7% of dogs weighing less than 15kg were found to be improved (10.7%) or clinically normal (75%) after an average follow-up of three years. Conversely, only 19.3% of the dogs weighing 15kg or more were found to be improved (12.3%) or normal (7%) after an average of 4.1 years. The author concluded the reason smaller dogs seemed to fare much better was likely linked to their less active lifestyle, the fact small breed dogs are generally kept as pets and CrCL rupture in small dogs tends to occur at an older age when their activity levels have already started to decline.
Vasseur’s findings are similar to those of an older study reporting close confinement for four to eight weeks led to a satisfactory outcome in 91% of dogs weighing less than 20kg versus 77% of dogs weighing more than 20kg (Pond and Campbell, 1972). The discrepancy in the outcome of heavier dogs between the two studies might be explained by the fact the latter study looked at a smaller sample of conservatively managed dogs (50) and had lameness evaluated by the owner, which could have biased results in favour of conservative management. However, neither study used objective measures of gait analysis and it is likely subtle lameness was overlooked in both reports.
While conservative management may lead to acceptable results in some cases, it is generally accepted dogs will be more likely to return to full function and will do so more quickly following surgical stabilisation. Many eminent surgeons report their clinical experience has led them to recommend surgical treatment of dogs with CrCL disease wherever practical (Piermattei et al, 2006).
However, surgical management is not possible in all cases. Other considerations – including age of the patient, concomitant conditions and financial restrictions – may preclude proceeding to surgery and necessitate proceeding with non-surgical management. In these cases, the authors recommend six weeks of strict rest with analgesia being attended to for as long as required, generally at least the initial 7 to 10 days. The prognosis for return to satisfactory function will depend on many factors, including the size and body condition score of the patient, whether the disease is unilateral or bilateral and whether concomitant meniscal injury is present.
Persistent meniscal impingement is likely to lead to a persistent, severe lameness and non-surgical management cannot realistically be expected to result in substantial improvement. Large-breed dogs with bilateral and severe instability are given a guarded to poor prognosis for return to function. Smaller-reed dogs with unilateral disease, and where strict exercise restriction can be enforced, may have a fair prognosis for return to satisfactory function.
In all cases, if satisfactory improvement is not observed after six weeks of appropriate conservative management, the prognosis for further improvement becomes guarded.
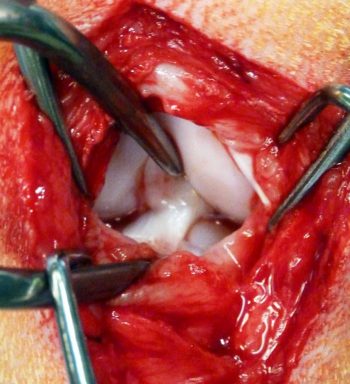
Regardless of the surgical technique used to stabilise the cruciate deficient stifle, surgery should always be preceded by joint exploration to visualise and assess intracapsular structures (Figure 1).
The joint capsule is entered either by arthroscopy or parapatellar arthrotomy. The CrCL is identified. In cases of complete tears, the remnants of the torn ligaments are typically debrided and integrity of the caudal cruciate ligament is systematically assessed. In cases with partial tears, whether the remaining band should be debrided is controversial.
It is the preference of the authors to debride remaining strands in an attempt to reduce ongoing inflammation and pain associated with a degenerative remnant. However, an argument exists that when the residual strand remains healthy, it should be left in situ to provide some stability – particularly against internal tibial rotation and stifle hyperextension, which many of the treatment methods described do not address. To the knowledge of the authors, no definitive evidence is available to support debridement or leaving the residual strand in place and, therefore, this decision remains at the discretion of the surgeon.
As mentioned in part two of this article (VT47.31), meniscal tears are common in the cranial cruciate deficient stifle and identified in 33.2% to 77% of dogs with CrCL pathology (Casale and McCarthy, 2009; Ralphs and Whitney, 2002; Williams et al, 1994; Fitzpatrick and Solano, 2010), with the vast majority of tears affecting the medial meniscus only (Flo and DeYoung, 1978).
Although, clinical signs – such as exacerbation of lameness in a CrCL deficient stifle and/or the presence of an audible click when ambulating and/or manipulating the stifle – are signs indicative of possible concomitant meniscal injury, a definitive diagnosis can only be made on observation of an abnormal meniscus (Case et al, 2008) and meniscal evaluation represents a crucial part of stifle joint exploration.
When a meniscal tear is evident, the entire caudal horn of the meniscus may be folded cranially and a diagnosis of meniscal injury obvious (Kowaleski et al, 2012). In cases where an injury is not immediately apparent, however, producing cranial tibial subluxation may help in evaluating meniscal stability; the caudal horn of the medial meniscus may become visible during drawer movement, which reflects fibres of the caudal horn slightly elevated off the tibia after having been crushed and separated. When this is observed, a partial meniscectomy is indicated (Piermattei et al, 2006). In cases where chronicity has resulted in significant periarticular fibrosis, however, drawer motion may be limited and observation of the caudal meniscal horn is more challenging.
Hence, in addition to looking for gross displacement, probing should always be performed to evaluate any region that cannot be observed. Active probing allows assessment of the integrity of the different meniscal attachments, as well as feeling for irregularities on the meniscal surface. Hooking or catching of the probe on the meniscus suggests the presence of a longitudinal non-displaced tear that can be manually pulled forward. Although injuries to the lateral meniscus are very rarely seen, it cannot be emphasised enough both menisci should always be equally assessed.
The most common canine meniscal injuries observed are a displaced bucket handle tear of the caudal horn of the medial meniscus and peripheral detachment of the crushed caudal horn of the medial meniscus (Piermattei et al, 2006; Fitzpatrick and Solano, 2010). The menisci have a rich nervous supply (O’Connor, 1976) and are typically painful injuries causing marked lameness. The inner two-thirds of the menisci are also avascular, with poor healing potential. This rich nervous supply and poor healing potential form the rationale for removal of any damaged meniscal tissue before restoring normal stifle joint biomechanics.
Meniscectomy can be partial (the axial torn portion of the meniscus only is resected and the peripheral rim preserved), segmental (a portion of meniscal tissue extending from the axial to the abaxial margins is removed) or total (complete meniscal resection; Pozzi et al, 2010). The menisci serve several functions within the stifle joint – their most essential role is to protect femoral and tibial articular cartilages by distributing compressive forces during weight bearing (Hulse and Shires, 1983; Williams et al, 1994).
Indeed, up to 65% of the compressive load is transformed into “hoop stresses” at the meniscal periphery, thus sparing the articular cartilage (Messner and Gao, 1998). The menisci further protect articular cartilage by contributing to its hydrostatic lubrication (Hulse and Shires, 1983). Additionally, they enhance joint stability by improving femorotibial congruity (Bennet and May, 1991).
The biomechanical importance of these meniscal functions is the rationale behind a conservative approach regarding the amount of meniscal resection that should be performed in presence of a meniscal injury. Resection should be sufficient as to remove all pathological tissue, while preserving as much normal tissue as possible to preserve meniscal function (Kowaleski et al, 2012).
In cases where stifle explore fails to reveal the presence of any meniscal injury, controversy exists regarding the best procedure to follow. Indeed, while general agreement exists that healthy meniscal tissue should be preserved as much as possible, postoperative meniscal injuries (such as meniscal tears developing after stabilisation of the cruciate deficient stifle) are well recognised. When evaluating three different CrCL extracapsular surgical stabilisation procedures, Metelman et al (1995) reported an incidence of latent meniscal tears of 13.8%. Similarly, the incidence of late meniscal tears following tibial plateau levelling osteotomy (TPLO) was found to be 6.3% in one study (Thieman et al, 2006).
It has been suggested late meniscal tears occur because of either an inability to correct all the abnormal forces present in the cranial cruciate deficient stifle and/or a failure to observe a meniscal injury that might have been present at the original surgery (Wheeler et al, 2003; Thieman et al, 2006). These postoperative tears have prompted some surgeons to consider performing a medial meniscal release by radial transection at either the caudal meniscotibial ligament of the medial meniscus or at the mid-body of the medial meniscus (Thieman et al, 2006; Kowaleski et al, 2012). This, theoretically, allows the caudal horn to move caudally, avoiding impingement between the medial femoral and tibial condyles (Kowaleski et al, 2012), hence eliminating the “wedge effect” described in part two.
Meniscal release, however, remains controversial. Indeed, in a retrospective cohort study evaluating 254 dogs that had received a TPLO, Thieman et al (2006) found meniscal release did not significantly reduce the rate of subsequent meniscal tears. Additionally, meniscal release by transection of the caudal meniscotibial ligament is thought to eliminate the load bearing function of the medial meniscus and predispose to further degeneration of the medial compartment after TPLO (Pozzi et al, 2010).
It is the preference of the authors not to perform a meniscal release in the presence of an intact meniscus. However, it is imperative owners are warned regarding the potential risks of late meniscal injury. In cases where owners would not be able/willing to proceed with a second surgery in the event of a late meniscal injury, the only way to completely prevent these future injuries is to perform a complete medial meniscectomy at the time of the original surgery. While absolutely not recommended in general, this may have to be considered in select cases.
Multiple surgical procedures have been described for the management of CrCL rupture. These can be divided into extracapsular, intracapsular and osteotomy techniques. It is beyond the scope of this review to present all of these procedures in detail and, in addition to this, some of them, including traditional intracapsular techniques, have been shown to lead to inferior results, therefore, becoming less commonly performed.
The remainder of this article will focus on the techniques most commonly performed, including extracapsular suture (ECS), tibial tuberosity advancement (TTA) and TPLO.
ECS techniques is an umbrella term representing a body of procedures where heavy-gauge suture material is used to increase joint stability by mimicking the action of the ruptured CrCL and limiting cranial drawer movement. The stability first provided by these techniques is short lived and long-term stability ultimately relies on the formation of periarticular fibrotic tissue within 8 to 12 weeks, postoperatively (Vasseur, 2003; Piermattei et al, 2006).
In their seminal publication, DeAngelis and Lau (1970) first presented the lateral retinacular imbrication technique, where one or two large non-absorbable sutures are placed around the lateral fabella and anchored to the distal patellar ligament, preventing drawer movement.
Building on this work, Flo first described the modified retinacular imbrication technique (MRIT) in 1975. As described by Piermattei et al (2006), in this technique monofilament nylon leader line mattress sutures are passed around both the lateral and medial fabellae and anchored to a hole drilled in the tibial tuberosity. The size of the suture approximates the weight of the animal. The lateral fabella is identified first and suture material passed behind it, through the femorofabellar ligament. The same step is repeated for the medial fabella.
Next, a horizontal hole is drilled in the tibial tuberosity. The distal end of the lateral strand is brought to the medial side by feeding it under the patellar ligament. This end is brought back to the lateral side by feeding it through the hole previously made in the tibial tuberosity. A mirror manoeuvre is applied to the medial fabella suture strand.
Once these sutures are preplaced, the lateral suture is tightened first to externally rotate the tibia and, therefore, limit internal rotation normally prevented by the intact CrCL. The medial suture is then tightened. Each suture can be secured using a series of square knots, a slipknot followed by a series of square knots, or a tensioning device and metallic tube crimps (Kowaleski et al, 2012).
While the choice of method used to secure this suture is left to the surgeon’s preference, an ex vivo biomechanical study found the crimp clamp method was found to have lower loop elongation and higher load to failure when compared with nylon leader line secured with a square knot (Anderson et al, 1998). McCartney et al (2007) retrospectively evaluated 110 dogs that received an extracapsular suture secure with the crimp cramp system and found a rate of failure due to premature slippage through the crimp of 8%.
Little information exists about the precise joint angle the sutures should be tightened at. Piermattei et al (2006) recommend finding the stifle angle where the most drawer movement is present and tightening the lateral suture with the leg held at this same angle while over-reducing the cranial drawer. However, an ex vivo study suggests tightening sutures at a joint angle of 100° might result in the best balance between preserving most of the tension within the suture when the joint is extended, but, at the same time, limiting stretch of the suture knot construct (Fischer et al, 2010).
While the original MRIT technique described makes use of nylon sutures on both the medial and lateral side, a common variation on this technique is the use of sutures involving only the lateral side – lateral fabellotibial suture (LFS) or modified Flo technique. Although the use of both a medial and lateral suture as originally described in the MRIT technique may be better at harnessing the tibia in place, the medial sutures put stress on the lateral ones by encouraging some degree of internal rotation, which has encouraged a gradual move from MRIT to the placement of a lateral suture only.
Although sutures on the lateral side adequately prevent cranial drawer, they do also completely eliminate internal tibial rotation and, hence, limit stifle physiological range of motion to an extent. This, however, appears to be of little clinical relevance.
A retrospective study aimed at reporting the rate and nature of the complications associated with LFS found complications arose in 17.4% of dogs and included surgical site infections, incisional complications (self-trauma, swelling and discharge, bandage related) and implant-related complications (infection and stifle instability due to premature breakage of the extracapsular suture; Casale and McCarthy, 2009). Of 363 performed, 26 (7.2%) required additional surgery (implant infections or failure in 5.3% of cases and meniscal complications in 1.9% of cases).
Dogs treated with an ECS should, ideally, follow strict exercise restriction during the first eight weeks, with a progressive return to activity over subsequent weeks (Piermattei et al, 2006). This is of crucial importance, as the implants in this technique only serve as temporary prostheses until sufficient periarticular fibrosis forms (typically 8 to 12 weeks postoperatively). Excessive stress on the nylon sutures is likely to cause implant failure.
In dogs that receive an ECS, physical rehabilitation should be considered part of the postoperative management. In a prospective study analysing the effects of postoperative rehabilitation on limb function after lateral suture stabilisation and medial meniscectomy, the group that consistently walked, swam and received hindlimb massages twice daily between week three and seven postoperatively had significantly higher peak vertical forces and vertical impulses (both reliable indicators of lameness in dogs) six months post-surgery (Marsolais et al, 2002).
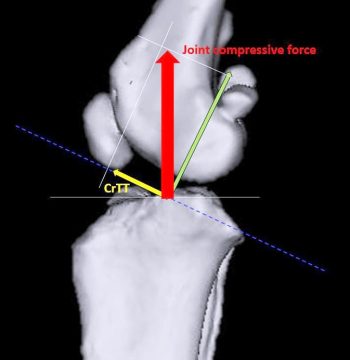
Slocum and Devine (1983) were the first to identify and describe a cranially directed force within the canine stifle joint.
According to their model, ground reaction forces created during the stance phase are transmitted along the axis of the tibia and a shear is generated by the compression of the femur against the tibial plateau. Because of the caudal direction the slope of the tibial plateau, axial compression between the joint surfaces results in an overall femorotibial shear force of cranial orientation – referred to as cranial tibial thrust (CrTT; Figure 2). Because of its orientation, CrTT leads to cranial translation of the tibia.
In a stable stifle, this translation is countered by an active force generated by the hamstring musculature and a passive restraint provided by the CrCL and menisci. In the presence of CrCL rupture, the ligament is no longer capable of counteracting the CrTT described previously, and the tibia slides forward in relation to the femur (Slocum and Devine, 1993).
The TPLO technique aims at providing functional stifle stability during the stance phase of the gait cycle by eliminating CrTT (Slocum and Devine, 1983, 1984, 1993; Slocum and Devine-Slocum, 1998). This represents a difference from intracapsular and extracapsular stabilisation techniques, which both aim at restoring stifle stability throughout the joint range of motion. Because the CrTT is a result of the tibial plateau orientation at an angle to the axial compressive forces described, Slocum hypothesised that levelling the tibial plateau, such that the tibial plateau angle (TPA) is reduced to 0°, would eliminate the CrTT, in turn providing functional stability to the stifle during weight bearing.
In a landmark ex vivo study, Warzee et al (2001) were the first to validate the mechanics of the TPLO. However, their work additionally proved rather than neutralising the CrTT, levelling the tibial plateau to 0.5° transformed CrTT into caudal tibial thrust, generating caudal tibial translation and increasing the stress on the caudal cruciate ligament (CaCL), in turn increasing risk of CaCL injury and rupture. The authors identified rotation of the tibial plateau to a postoperative TPA of 6.5° provided stifle stability and eliminated CrTT, while considerably reducing the stress put on the CaCL. Clinically, a final TPA of 5° to 6° is recommended after TPLO (Piermattei et al, 2006).
The patient’s TPA is determined preoperatively and the rotation needed to achieve an end point TPA of 5° to 6° calculated using a TPLO rotation reference chart. A curved osteotomy in the proximal tibia is performed using a biradial saw blade. A threaded pin is temporarily placed in the proximal segment to assist with rotation. The proximal tibial fragment is rotated to the appropriate angle and temporarily stabilised in that position using a further threaded pin, while the TPLO plate is placed to provide medium-term stabilisation pending osseous union (Figure 3).
Complications reported after TPLO include incisional complications, infection, tibial tuberosity, fibular or tibial fractures and implant failure (screw loosening or breakage; Priddy et al, 2003; Pacchiana et al, 2003; Stauffer et al, 2006). Earlier studies report overall complication rates ranging from 18.8% to 28%, which are likely to partly reflect the steep learning curve associated with this procedure (Priddy et al, 2003; Pacchiana et al, 2003; Stauffer et al, 2006).
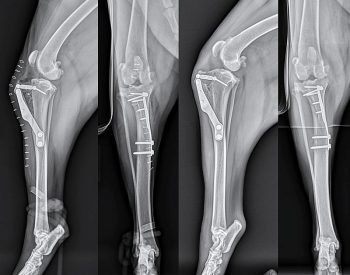
A study performed on 1,146 cranial cruciate deficient stifles stabilised with TPLO identified an overall complication rate of 14.8%, 6.6% of which were major complications (Fitzpatrick and Solano, 2010). Major complications included plate removal with or without positive microbial culture, tibial tuberosity fracture/avulsion, medial patellar luxation and broken screws. Despite this complication rate, 93% of owners have been reported to be satisfied following TPLO (Priddy et al, 2003).
It is important to note because the TPLO technique only provides joint stability during the stance phase of the gait cycle, a positive direct drawer test is to be expected postoperatively if the cranial cruciate ligament tear was complete, or if the remnants were resected. Instability should not be evident in cranial tibial thrust or indirect drawer, however, as this test mimics the mechanics of weight bearing (Slocum and Devine, 1993).
Like the TPLO technique, TTA is a commonly performed osteotomy technique that aims to neutralise CrTT. The goal of TTA technique is to move the insertion of the patellar ligament cranially so the patellar ligament and the articular surface of the tibia are at 90° to each other. The rationale behind TTA is originally based on a biomechanical model analysis of the joint forces occurring in the human knee which demonstrated the CrTT force was approximately of the same magnitude and direction as the patellar tendon force, which is determined by the patellar tendon angle (PTA; Nisell et al, 1986).
In the dog, it was suggested a PTA of 90° would neutralise thrust and, additionally, a PTA greater than 90° would result in cranial thrust, while a PTA less than 90° would result in a caudally directed thrust (Montavon et al, 2002; Tepic et al, 2002; Tepic and Montavon, 2004). This proof of concept was demonstrated in an in vitro experimental biomechanical study that showed advancement of the tibial tuberosity neutralised cranial tibial thrust at a PTA of 90.3°+ / −9° (Apelt et al, 2007). Since this, several clinical studies have proven TTA to be a valid treatment modality for the cranial cruciate deficient stifle, with good to excellent outcomes and return to function reported in the vast majority of cases (Hoffman et al, 2006; Lafaver et al, 2007).
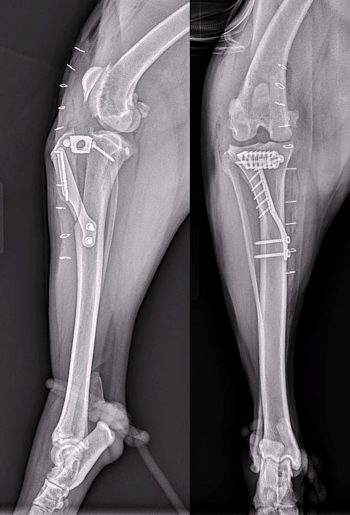
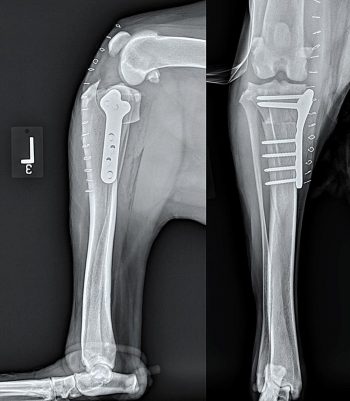
An oscillating saw is used to free the tibial tuberosity via a frontal plane osteotomy. A metal cage is placed at the proximal aspect of the osteotomy to advance the tibial tuberosity and this is held in place using two screws. The cages are available in different widths, which allows the amount of advancement to be tailored to the patient based on preoperative templating to create the appropriate PTA. A plate that acts as a tension-band is placed from the tibial tuberosity to the tibial diaphysis to stabilise the tibial tuberosity in the new position. The plates are held in place using either screws or tines in the tibial tuberosity (depending on the manufacturer of the implant) and screws in the tibial diaphysis. A bone graft may be placed at the osteotomy site depending on the surgeon’s preference (Figure 4).
The first two studies evaluating TTA reported an overall complication rate of 31.6% to 59%. Major complications encountered included, in order of frequency: late meniscal tears (7.3%); infection (3.9%); MPL (1.1%); tibial fracture (1.1%); and catastrophic implant failure (1.1%). A revision rate of 11.3% was reported (Lafaver et al, 2007). The high overall complication rates reported included a number of minor complications, such as bruising and swelling, and did not necessarily reflect the clinical outcome associated with TTA.
The most frequent complication observed was late meniscal tears, which are a known complication associated with the CrCL deficient stifle and are likely not dependent on the choice of method for surgical stabilisation, as previously discussed. Although, initially, the rate of late meniscal injury appeared to be higher following TTA than that reported following TPLO, later studies have not supported this finding. Radiographic healing is reported to occur between 8 to 10 weeks postoperatively for TTA (Figure 4).
A later study evaluating TTA in 458 stifles found an overall complication rate of 19% with a major complication rate (defined as fracture, implant failure, any complication requiring revision surgery and lameness for which the underlying cause could not be determined) of 11.4% (Wolf et al, 2012). Overall incidence of fracture was 4.2% and a 2% incidence of implant failure. Again, a greater number of cases overall and increased experience with the procedure may explain why the complication rate in this later study was lower than the one observed in the aforementioned earlier reports.
TTA was first proposed as an alternative to TPLO in 2002 (Montavon et al, 2002) and has become popular in recent years. Experience and technique are likely to play a role in both fracture and implant failure cases, hence higher complication rates reported in earlier studies are likely to reflect technical errors associated with the learning curve that accompanies any new procedure. Poor plate and/or cage positioning and narrow distal osteotomy width have both been identified as risk factors for tibial tuberosity fracture after TTA in dogs (Nutt et al, 2015), and are both directly related to surgical experience and technique.
As with the TPLO technique, and for the same biomechanical reasons, a positive direct drawer test, but negative indirect drawer test (which mimics the mechanics of weight bearing), are to be expected postoperatively.
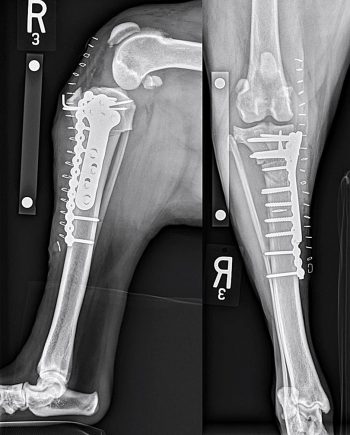
With many surgical options available to stabilise the cranial cruciate deficient stifle, the question of the potential superiority of one technique over another arises. While the inferiority of techniques such as fibular head transposition and intracapsular stabilisation has been demonstrated (Chauvet et al, 1996; Conzemius et al, 2005), studies are yet to show the existence of clear differences in outcome and complication rates between ECS, TPLO or TTA. Ultimately, with the exception of a few particular scenarios, the choice of technique depends on a surgeon’s preference and experience.
In a 1999 survey, diplomates of the American College of Veterinary Surgeons preferred the MRIT over all other techniques for stifle stabilisation in both small (less than 16kg) and large dogs (Leighton, 1999). Although osteotomy techniques are typically performed in larger dogs, it should be noted nothing actually precludes the use of an ECS in larger dogs, and use of an ECS has been reported in dogs as heavy as 54.1kg (Lazar et al, 2005). Conversely, both TPLO and TTA have been performed in dogs as small as 5.4kg (Fitzpatrick and Solano, 2010; Wolf et al, 2012). Both TTA and TPLO rely on the presence of an intact CaCL able to passively limit the caudal thrust typically associated with these technique and, as such, TTA and TPLO are methods that cannot be used to repair traumatic CrCL associated with CaCL rupture.
While the originators of the TPLO technique have consistently cited an earlier and better return to function over other surgical methods, this was only evidenced by a prospective clinical trial aiming at comparing the long-term outcome of TPLO versus ECS in dogs suffering from CrCL deficiency. Gait analysis was performed on 15 dogs that received a TPLO and 23 dogs that received an ECS, and found dogs did achieve normal limb loading faster after TPLO when compared to ECS (Nelson et al, 2013). However, it is interesting to note the ECS group in this study did not undergo a stringent rehabilitation programme, which has been shown to be key to return to function in ECS repair as discussed (Marsolais et al, 2002).
In cases of concomitant CrCL rupture and MPL, the TTA technique may present an advantage over TPLO since simple lateralisation of the tibial tuberosity, bending of the plate and insertion of a spacer under the cranial ear of the cage allow lateral translation of the tibial tuberosity in addition to advancement, allowing simultaneous realignment of the quadriceps mechanism (Figure 5; Yeadon et al, 2011).
Cases with excessive tibial plateau slope may not be conducive to TTA because they are likely to require advancement of the tibial tuberosity beyond what can be obtained with available implants. It has been suggested dogs with a TPA greater than 30° may not be candidates for TTA (Boudrieau, 2009). However, dogs with excessive TPA (classified as more than 33°) may also not be candidates for routine TPLO; in these more complex cases, combination approaches are often required, with the most common approach being the combination of a cranial closing wedge osteotomy with a TPLO (Figure 6).