4 Aug 2020
Chris Linney and João Neves detail this case from the asymptomatic phase to the clinical phase, and present a rationale for such diagnoses.
Mitral valve disease (MVD) is the most common cardiac condition in dogs and represents approximately 75 per cent of all cases of cardiac disease.
Following recent groundbreaking MVD research, it is recognised that intervention prior to the development of congestive heart failure (CHF) in the presence of cardiomegaly can improve survival times and delay the onset of CHF.
This report follows a case of MVD from the asymptomatic phase through to the clinical phase, and presents a diagnostic and therapeutic rationale for these cases in line with the new American College of Veterinary Internal Medicine (ACVIM) MVD staging (Panel 1) and guidelines1.
Stage A
At risk of developing heart failure, but without apparent structural abnormality.
Stage B1
Asymptomatic dogs with mitral regurgitation caused my MVD that is not severe enough to meet the criteria used to trigger medical treatment to delay the onset of heart failure (see B2 criteria).
Stage B2
Asymptomatic MVD causing mitral regurgitation severe enough to result in left atrium and left ventricle enlargement sufficient to recommend treatment before the onset of clinical signs:
Stage C
MVD severe enough to cause current or past clinical signs of heart failure.
Stage D
MVD with clinical signs of heart failure refractory to standard treatment. Patients require more than a total daily dose of 8mg/kg of furosemide (or torasemide equivalent), administered concurrently with standard doses of the other medications thought to control clinical signs of congestive heart failure (pimobendan, benazepril and spironolactone).
Kiara – an eight-year-old 8.2kg female, neutered cross-breed – was diagnosed in February 2016 with early preclinical/asymptomatic MVD, classified as ACVIM stage B1 (absence of clinical signs and no radiographic or echocardiographic evidence of cardiomegaly) following investigations of an asymptomatic 4/6 left apical systolic heart murmur.
Reassessment 15 months later (May 2017) revealed the patient to be asymptomatic; however, Doppler echocardiography showed evidence of left atrial and ventricular dilatation (left atrial to aortic root ratio [LA:Ao] 1.7, reference interval [RI] less than 1.6; normalised left ventricular internal dimension in diastole [LVIDdN] 2.17, RI less than 1.7), compatible with progression to MVD ACVIM stage B2 (absence of clinical signs with cardiomegaly).
As echocardiographic criteria for introduction of pimobendan before onset of clinical signs (as recommended by the EPIC trial2) were met, the patient was started on pimobendan (0.2mg/kg twice a day). In addition to this, the owners were asked to monitor the resting respiratory rate (RRR) on a daily basis with commercially available and free monitoring software.
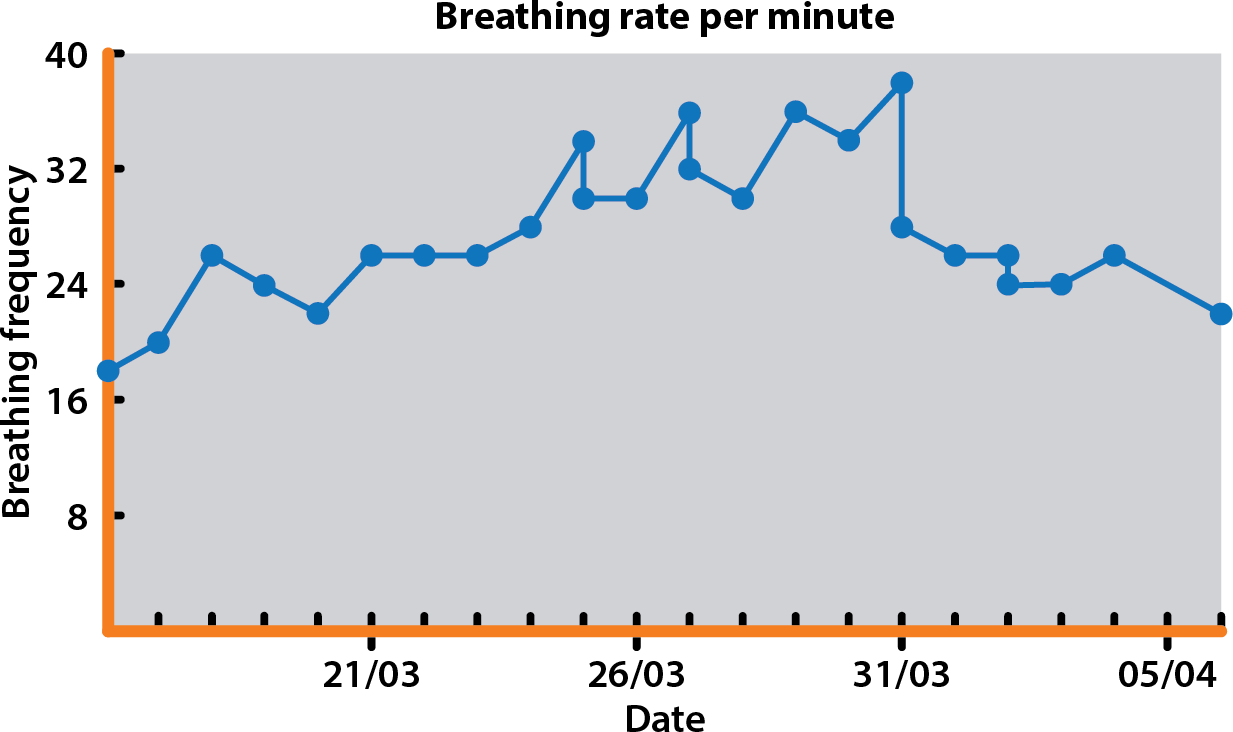
In March 2019 (22 months into ACVIM stage B2), the owners reported a gradual increase in RRR from 18 breaths/min to values more than 30 breaths/min over several days (Figure 1).
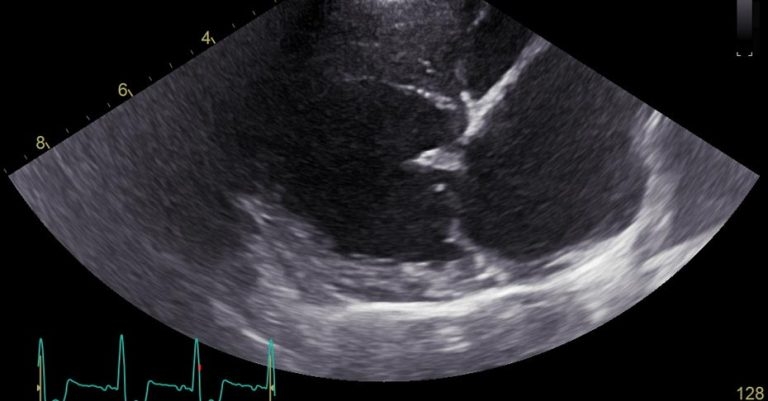
On presentation, the heart murmur had increased in intensity (grade 5/6) and mild tachycardia was present (160bpm), with a mild increase in respiratory effort and a RRR of 40 breaths/min. The rest of the physical examination was unremarkable.
Thoracic radiographs (Figure 2) showed a diffuse, mild interstitial pattern throughout the caudal lung fields, which, combined with the echocardiographic findings (Figure 3; LA:Ao 2.2, LVIDdN 2.44, dilated pulmonary veins, bulging of the interatrial septum into the right and increased left-sided filling pressures, along with a newly detected severe septal leaflet prolapse), suggested early onset left-sided CHF with mild pulmonary oedema.
The patient was hospitalised over the course of the day for IV furosemide therapy – this rapidly resulted in a normal respiratory rate and effort after two boluses of 2mg/kg IV given two hours apart. She was discharged with oral pimobendan (0.25mg/kg twice a day), benazepril (0.3mg/kg once a day) and spironolactone (2.7mg/kg once a day) tablets and furosemide (2.7mg/kg three times a day).
Prior to and seven days post-discharge, systolic blood pressure, as well as blood urea and creatinine, were within the normal limits. The owners were instructed to continue to monitor the RRR with the monitoring software, which allowed titration of the furosemide dose one week post-discharge to 5.3mg/kg/day.
The patient remained stable until mid-May 2019 when the RRR started to be occasionally more than 30 breaths/min (Figure 4), which prompted an increase in the oral furosemide dose – this resulted in a stabilisation of RRR around 20 breaths/min.
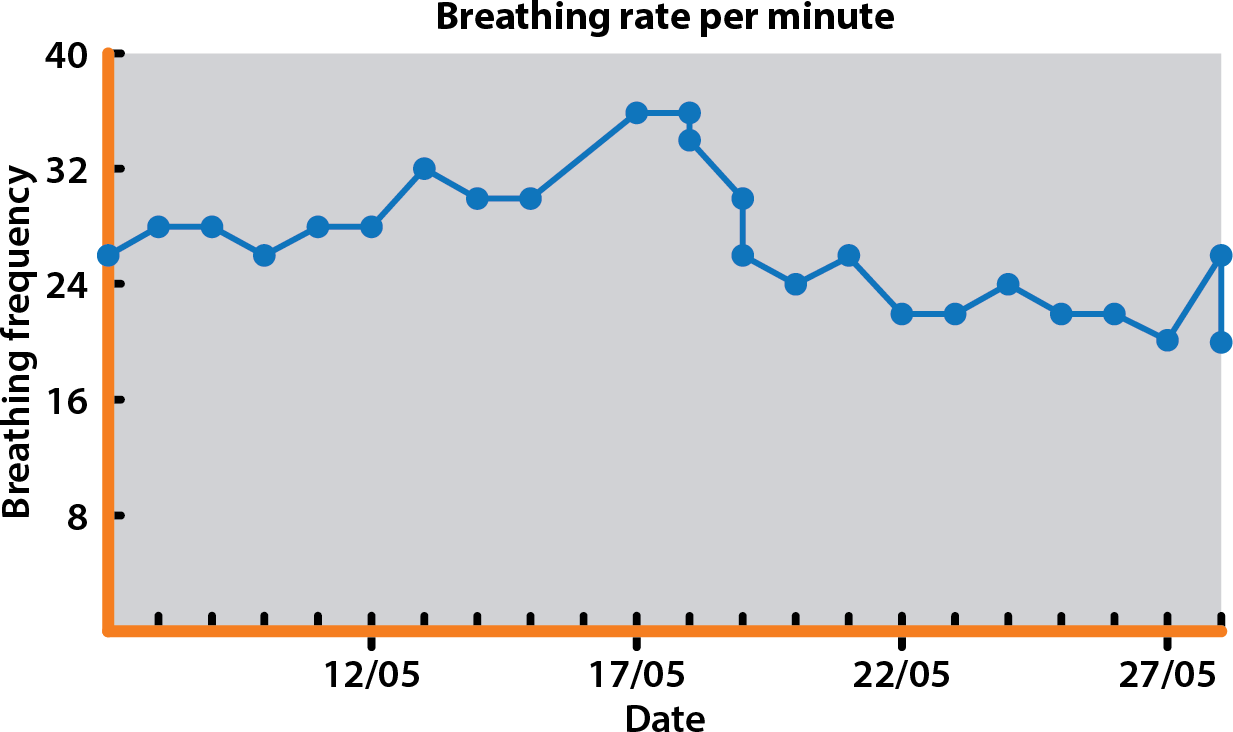
This case highlights the usefulness of monitoring the RRR in cases of MVD in ACVIM stage B2 (patients with radiographic and/or echocardiographic evidence of left-sided heart enlargement). The owner’s close monitoring at home allowed an increasing RRR to be identified and allowed intervention in early CHF rather than the point of severe, peracute pulmonary oedema.
These cases have haemodynamically significant valvular regurgitation, and predicting onset of CHF may prevent a peracute or emergency presentation, which can be very stressful for patient, owner and clinician.
Additionally, a severely unstable dyspnoeic patient may preclude the immediate use of some diagnostic tests (for example, baseline thoracic radiographs, echocardiography or even blood tests) that could be otherwise employed in a relatively stable patient with early onset pulmonary oedema.
In this case, the gradual increase in the sleeping respiratory rate to values more than 30 breaths/min raised the suspicion of imminently or early left-sided CHF. This was confirmed with the radiographs taken on admission. Importantly, having a baseline RRR allows the owner and clinician to closely monitor for an increasing rate, and intervene at an earlier stage.
The initial stabilisation was achieved with IV furosemide and oral pimobendan during a short period of hospitalisation. The initial furosemide administration could have also been given SC as peak urinary output is similar with IV and SC administration, and occurs by both methods around one hour post-injection3.
Therefore, an SC route may be an effective means for administration of furosemide in dogs – particularly if they are aggressive, in very early stages of heart failure or where IV access is difficult.
IV pimobendan could have replaced the oral route during hospitalisation – its cardiovascular effects (inotropism and decrease in left ventricular filling pressures) are readily seen in five minutes4. This rapid effect is particularly useful in very unstable CHF patients where a rapid decrease in filling pressures is crucial.
However, oral pimobendan is often sufficient in many cases that are relatively stable as its peak plasma concentration and peak cardiovascular effects can occur in 1.1 hours and 2 hours post-administration, respectively, based on a pharmacokinetic study of nonaqueous pimobendan oral solution.
The patient was discharged on oral pimobendan, furosemide, and benazepril and spironolactone. The QUEST trial showed that pimobendan is superior to benazepril in stage C (stage with clinical signs of heart failure) in prolonging survival time5. However, lack of a powerful study exists assessing the potential benefit of using pimobendan, benazepril and spironolactone together.
Despite this, and considering the theoretical benefits of antagonising the angiotensin II (ATII) and aldosterone, the new ACVIM consensus guidelines for diagnosis and treatment of MVD recommend the concomitant use of furosemide, pimobendan, angiotensin-converting enzyme (ACE) inhibitors and spironolactone as a mainstay of home treatment of stage C MVD.
Benazepril is an ACE inhibitor that reduces ATII (and its maladaptive cardiac effects), whose action can be particularly important in maintaining glomerular filtration rate in patients with poor renal perfusion/aggressive diuresis.
Therefore, although benazepril is commonly added to pimobendan and furosemide at discharge (as in this case), the timing for its introduction in patients with borderline hypotension and renal dysfunction may be delayed until after initial stabilisation.
In these cases, benazepril is frequently added to the classic heart failure therapy secondary to MVD only after confirming that the patient is not significantly hypotensive and azotaemic 3 to 10 days after starting oral furosemide.
Spironolactone is an aldosterone antagonist with weak potassium-sparing diuretic properties. Its use is mainly aimed to reduce the myocardial fibrosis and smooth vascular muscle remodelling associated with CHF and ATII.
It can cause hyperkalaemia, but this potential side effect is offset by the kaliuresis promoted by furosemide, and while theoretically hyperkalaemia needs to be considered, the authors have never encountered this clinically with quadruple therapy.
The home medical management of ACVIM stage C MVD is greatly aided by the monitoring of RRR. Whereas RRR at or below 30 breaths/min does not suggest the presence of pulmonary oedema on radiographs, a sudden increase in RRR or values that exceed 40 breaths/min should prompt attention from the clinician, even in patients with equivocal thoracic radiographs.
The home monitoring of the RRR, apart from predicting recurrence of pulmonary oedema, also allows clinicians to more accurately tailor home furosemide dosing to a target respiratory rate and to avoid excessive or insufficient diuresis. Therefore, the owners should be taught how to count the breathing rate with or without the help of a smartphone app.