7 Mar 2022
Yeon Joon Park BVetMed, MRCVS recounts the presentation, diagnosis, treatment and outcome for a domestic shorthair with a swollen right axilla.
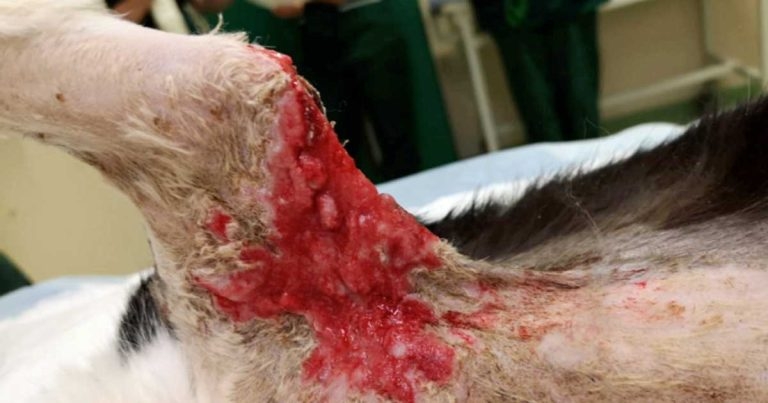
Smudge – a seven-year-old, male, neutered domestic shorthair – was presented with a week’s history of swollen right axilla with some purulent discharge. He was known to be an avid hunter and had a history of cat bite-related injuries.
Smudge had been eating, drinking, urinating and defecating without any problems, and had no other known illnesses. He was also fully vaccinated against feline herpesvirus-1 (FHV-1), feline calicivirus, feline panleukopenia virus and FeLV.
While a wound was visible, closer examination was not possible due to fractious temperament, so he was sedated to have a better assessment of the lesion.
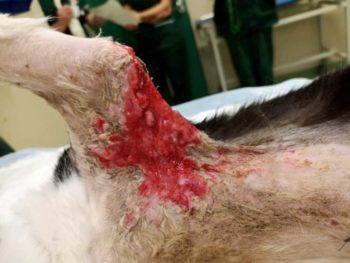
Physical examination under sedation revealed pale mucous membrane with capillary refill time of two seconds. Both thoracic auscultation and abdominal palpation revealed no abnormalities. The entire right fore was observed to be slightly swollen, and matching puncture wounds were visible on the axilla and on the elbow, with severe bruising in surrounding tissue. Raised, erythematous pustular lesions were also observed, and were noted for their thickened feeling on palpation (Figure 1).
In light of the history, Smudge was suspected to have been bitten while roaming outside, with possible secondary cellulitis. He was hospitalised with antibiotics and analgesia, with the view of monitoring for skin necrosis.
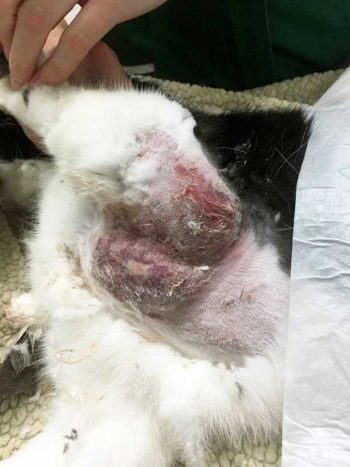
Despite treatment with antibiotics, however, no signs of improvement existed over five days. As expected, the skin in the affected area turned necrotic, and the surrounding tissue became even more elevated and erythematous (Figure 2). Multifocal, crusting, ulcerative lesions also appeared on both front feet and around the eyes.
In light of the progression of skin lesions despite antibiotic treatment, the differential diagnosis included:
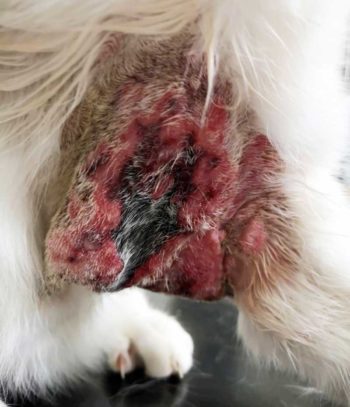
As cowpox was heavily suspected in light of history and presentation, skin biopsies were taken at multiple sites for histology and bacterial culture and sensitivity (Figure 3).
Histopathological examination of the cat’s skin revealed an ulcerative and necrotising dermatitis and folliculitis with pathognomonic eosinophilic intracytoplasmic viral inclusions consistent with feline cowpox virus infection. The samples were also tested positive for pox on PCR.
Cowpox virus (CPxV) is an orthopoxvirus indigenous to Great Britain. Although a wide range of mammals – including elephants, rhinoceroses, okapis, alpacas and cheetahs – have been known to be infected with the virus (Baxby et al, 1982; Pilaski and Rösen-Wolff, 1988; Prkno et al, 2017) cats are by far the most commonly affected (Bennett et al, 1990).
Bovine infection of CPxV is, ironically, actually rare (Baxby, 1977). Serological studies have demonstrated that bank voles, wood mice and short-tailed field voles serve as reservoir hosts in Great Britain (Chantrey et al, 1999; Crouch et al, 1995).
Affected cats often live in rural or suburban environments and are known hunters. An epidemiological study in Germany revealed that 83% of feline CPxV infections took place in between August and November (Appl et al, 2013), the same period when a seropositive proportion of bank voles and wood mice for CPxV reaches more than 70% (Chantrey et al, 1999).
Initial lesions have been described as small bite-like wounds, but do vary from small scabby lesions to large abscesses; 7 to 10 days later, secondary lesions start to appear, forming into discrete, circular, pruritic ulcers or papules of 0.3cm to 1cm in diameter with hard margins. These are subsequently covered by scabs and crusts (Gibbs, 2021; Möstl et al, 2013).
Some other clinical findings may include (with prevalence):
Although rare, fatal necrotising bronchopneumonia can also develop in the absence of skin lesions (Smith and Sloan, 2017).
Evidence of antibody with accompanying characteristic skin lesions is strongly indicative of CPxV infection. All suspected CPxV infections should be confirmed by virus isolation (Bennett et al, 1990).
Diagnosis of some poxvirus infections can be made based on clinical features and case history alone. However, laboratory testing is needed to confirm infection. Because large quantities of the virus can be found within lesions, vesicle fluid, crusts, or tissue biopsy are preferred as diagnostic samples (Tack and Reynolds, 2011).
Serum assays – including virus neutralisation, haemagglutination inhibition, complement fixation and ELISA – can detect antibody to orthopoxvirus (Bennett et al, 1990). PCR is also a confirmatory test for CPxV infection (Meyer et al, 2004).
The Poxvirus PCR can be run on scabs and tissues. Cats are infectious until the scabs have healed. In this case, CPxV-associated dermatitis was confirmed by PCR from skin punch biopsies. The negative PCR post-treatment suggested that the cat was no longer actively infected. Unfixed scabs or small quantities of exudate may also be used to identify the typical brick-shaped virions using negative-stain electron microscopy (Möstl et al, 2013).
Poxviruses are epitheliotropic; most infections are self-limiting and produce localised lesions that progress through macular, papular, vesicular and pustular stages over several weeks.
Most cats present with a generalised papular or vesicular rash that may have started as a single bite-like lesion around the head, neck or forelimb approximately one to two weeks prior. Approximately 20% of infected cats will develop oral lesions, and 20% will have a mild upper respiratory tract infection with serous to mucopurulent nasal and ocular discharge similar to what is seen with calicivirus infections and feline herpes (Tack and Reynolds, 2011).
Fatal outcomes in domestic cats have primarily been associated with underlying disease. Prognosis is generally good, with about 50% of cats completely healed within four weeks (Appl et al, 2013). Corticosteroids should be avoided at all times (Gibbs, 2021; Möstl et al, 2013).
It is important to note that while cat-to-human transmission of cowpox is rare, it has been documented numerous times (Eder et al, 2017; Schulze et al, 2007; Świtaj et al, 2015). In fact, a veterinary nurse at the author’s practice who was heavily involved in caring for another cowpox patient developed a circular, nodular, ulcerated skin lesion on her finger, followed by two weeks of general illness. Even though virus isolation was not conducted to confirm zoonotic transmission of cowpox, a high index of suspicion existed, given the circumstances (Smith and Sloan, 2017).
One immunosuppressed individual – an 18-year-old man on corticosteroids – has even died as a result of lung embolism in the course of intensive medical therapy for cowpox transmitted from a cat (Czerny et al, 1991). It is imperative, therefore, that all personnel involved in the care of a suspected cowpox patient are informed of these risks, and good hygiene measures with proper disinfection protocol should be implemented (Möstl et al, 2013).
The primary aim of management of cowpox infection is to treat the primary ulcer and to minimise secondary infections. Even though the diagnosis of cowpox was confirmed, significant challenges existed to manage Smudge’s case.
As one of the main aims for the management of feline cowpox infection is to minimise secondary infections, it is important to provide broad-spectrum coverage against all major bacterial pathogens associated with skin infections. These may include cefalexin, amoxicillin clavulanic acid, clindamycin and lincomycin.
Cefovecin can be used first line if administration or compliance were to be significant problems. Second-line antibiotics such as enrofloxacin, marbofloxacin and pradofloxacin should only be considered when there is culture evidence suggesting poor response to first-line antibiotics (Beco et al, 2013).
Initial antibiotic therapy for Smudge included first-generation and third-generation cephalosporin cephalexin, and cefovecin, respectively. When poor response to initial treatment was evident, however (Figure 3), bacterial culture and sensitivity test was performed. Concurrently, marbofloxacin was empirically added while pending results.
Culture and sensitivity demonstrated a profuse growth of Escherichia coli resistant to cephalexin and cefovecin, and Enteroccoccus faecalis resistant to tetracycline and doxycycline, providing some insight to the poor response to initial treatments. When oral medication could be resumed with clinical improvement, marbofloxacin was swapped to pradofloxacin.
Although Smudge was covered with lesions over his body, the “primary” lesion on his right axilla required the most attention.

Not only did the lesion contain significant amount of necrotic skin, but the severity of surrounding inflammation also produced significant amount of exudate leading to a state of hypoalbuminaemia (20g/L [reference interval 22g/L to 40g/L]; Figure 3).
A number of different wound management options were considered, including surgical debridement, but most were deemed impractical, given the location and Smudge’s temperament. The lesions were far too multifocal, extensive, and heavily infected for honey and silver-impregnated dressings.
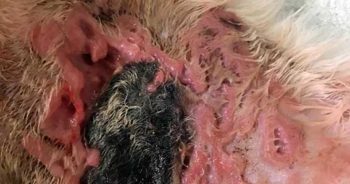
Negative-pressure wound therapy may have been an appropriate choice, but the extent of the lesion across the elbow and the axilla made this practically unrealistic. In the end, daily wet-to-dry bandages were used for rapid debridement of necrotic tissue and subsequent promotion of granulation tissue production. Once healthy granulation tissue was achieved (Figure 4), non-adhesive dressings were used to protect the lesions from self-trauma.
Smudge managed to return home sound after 45 days of hospitalisation. As zoonosis was a significant consideration, repeat skin biopsies were taken; all samples tested negative for pox virus PCR. As of day 52 post-presentation, no new lesions have appeared since discharge (Figure 5).
Feline cowpox infection is a potentially underdiagnosed condition that often causes multiple skin lesions on mostly outdoor cats. Due to its zoonotic potential, delays in diagnosing poxvirus-associated infections in companion animals can lead to inadvertent human exposures.
With appropriate antibiotic therapy, the lesions are often self-limiting, but intensive management may be required in cases with significant secondary infections or large lesions. Owners and all personnel involved in the care should be alerted of
the risks.
The author would like to thank Katharine Sloan and Georgina Mourant for their clinical and diagnostic support, and Valeria Cafe Marcal (Diagnostic Pathology Laboratory, Dick White Referrals) for her help in providing definite diagnosis.