25 Jun 2024
Karen L Perry focuses on three causes of pelvic limb lameness in cats.

Mediolateral and craniocaudal radiographs of the left stifle of a seven-year-old domestic shorthair cat, which presented with cranial cruciate ligament rupture. The stifle was stabilised with a lateral fabellotibial suture using a suture anchor placed in the caudal aspect of the lateral femoral condyle and a bone tunnel just proximal to the tibial tuberosity as the paired attachment points.
Sushi, an 18-month-old, male neutered Maine coon, presents for assessment of unilateral left pelvic limb lameness of three days’ duration. No known history of trauma exists.
Prior to the onset of lameness, with hindsight, the owner feels the cat might have been jumping up on to counters less often. The lameness is noted to be slightly worse in the mornings and after rest, but is not noted to deteriorate or improve with exercise. No medications or supplements have been trialled at this time.
Gait assessment shows a moderate left pelvic limb lameness with an associated hip hike and tail shift. The cat is tense, and only allows a partial orthopaedic examination without becoming stressed. Salient findings include reduced weight bearing on the left pelvic limb at stance and mild muscle atrophy affecting the proximal left pelvic limb musculature. A consistent pain response is present upon full extension of the left coxofemoral joint and an inconsistent pain response upon full extension of the left stifle. The thoracic limbs and right pelvic limb appear to be within normal limits. The remainder of the examination was delayed to be performed under sedation to avoid over-stressing the patient.
This is not an uncommon scenario to encounter in feline orthopaedics, but even with this limited database, it is necessary to establish possible differential diagnoses and propose a diagnostic plan to confirm or refute these.
In this article, we will discuss the three most common feline orthopaedic conditions that could present in such a manner, how these would be differentiated and the treatment options available for each.
Cranial cruciate ligament (CrCL) rupture is reported far less commonly in cats than in dogs. Potential explanations for this include the CrCL being larger than the caudal cruciate ligament in cats (Harasen, 2005; Umphlet, 1993), the lower amount of differentiation of fibrocartilage in the cat (Wessely et al, 2017) and the potential for many cats with CrCL rupture being treated conservatively; therefore, never being evaluated for lameness.
Historically, a traumatic event was assumed to be the main cause for CrCL rupture in cats (McLaughlin, 2002; Matis et al, 2010); however, the actual cause is not entirely clear in every case (Harasen, 2002; Matis et al, 2010).
The percentage of cats with clear evidence of a traumatic inciting injury varies widely from 16% to 80% (Scavelli and Schrader, 1987; Tacke et al, 1995). Additionally, the more recent literature contains an increasing number of reports detailing CrCL rupture in indoor cats (Harasen, 2005; Perry and Fitzpatrick, 2010); overview of these cases reveals some parallels with the reports of degenerative CrCL rupture in canines, in that these cats tend to be older, heavier and can be affected bilaterally.
Indeed, one study showed that up to 14% of cats appear to develop bilateral disease (Boge et al, 2020). In addition, although one study failed to confirm any histological evidence of degeneration as a contributing factor to CrCL rupture (Wessely et al, 2017), other studies have demonstrated the presence of degenerative changes such as calcification in the CrCL prior to complete rupture (Voss et al, 2017), and moderate to severe stifle osteoarthritis (OA) despite an acute onset of lameness (Mindner et al, 2016). These latter findings would tend to support a degenerative process in at least a subset of cats with CrCL injury.
Diagnosis of CrCL rupture is generally straightforward based on clinical signs, including lameness, stifle pain, crepitus, effusion and laxity in cranial drawer and cranial tibial thrust. Partial tears have been reported, although they are less common in cats, and these may be more challenging to diagnose, although, pain on stifle manipulation – particularly hyperextension – and moderate-marked lameness is generally evident. When assessing for partial tears, it is worth assessing for cranial drawer throughout the full range of motion (ROM) of the stifle.
With complete tears, abnormal cranial drawer motion will be evident throughout the full ROM of the stifle. For partial tears involving the craniomedial band in isolation, cranial drawer will only be evident in flexion, as the caudolateral band will prevent drawer when the stifle is extended. For isolated tears of the caudolateral band, cranial drawer motion will not be evident, as it will be controlled by the craniomedial band in both flexion and extension.
So, CrCL failure would explain the left pelvic limb lameness, the muscle atrophy and the pain noted on full extension of the left stifle for Sushi, but can we explain the pain response noted upon full extension of the left coxofemoral joint?
Here, we need to be aware of the potential for false positive pain responses during our orthopaedic examination. It can be challenging to fully extend the coxofemoral joint without also fully extending the stifle joint; as such, apparent pain on coxofemoral joint extension can be due to stifle pain, so, this is a potential explanation for Sushi.
One way to attempt to differentiate between these two is to perform coxofemoral joint abduction. This can be performed without extending the stifle joint and, as such, may enable you to isolate pain to either the stifle or the coxofemoral joint accordingly.
Should a sedated orthopaedic examination demonstrate instability in cranial drawer and cranial tibial thrust, our next step would likely be orthogonal radiographs of both stifles. When we think about stifle radiographs, in cats, it is common to see mineralisation within the articular space on the mediolateral stifle view. This often appears to be within the cranial pole of the medial meniscus and may represent a degenerative calcification.
Although meniscal mineralisation has not been associated with increased pain scores in cats, the presence of mineralisation has been associated with articular cartilage degeneration within the joint (Freire et al, 2010). However, in some cases, this mineralisation appears to be clinically insignificant and is thought to represent a meniscal sesamoid bone, the lunula (Whiting and Pool, 1984).
Differences appear to exist between small and large mineralisations with regard to concurrent OA and CrCL degeneration, with only the larger mineralisations being associated with stifle OA and degenerative changes of the CrCL (Voss et al, 2017). Small mineralisations tend to be located within the cranial horn of the medial meniscus and are well-circumscribed calcified bodies surrounded by otherwise normal or near-normal appearing meniscal fibrocartilage.
Large mineralisations tend to be ossifications consisting of mature cancellous bone, which originate at the cranial border of the medial meniscus or within the joint capsule in some specimens, or are located within the fat pad without evident connection to the menisci in other cases (Figure 1).
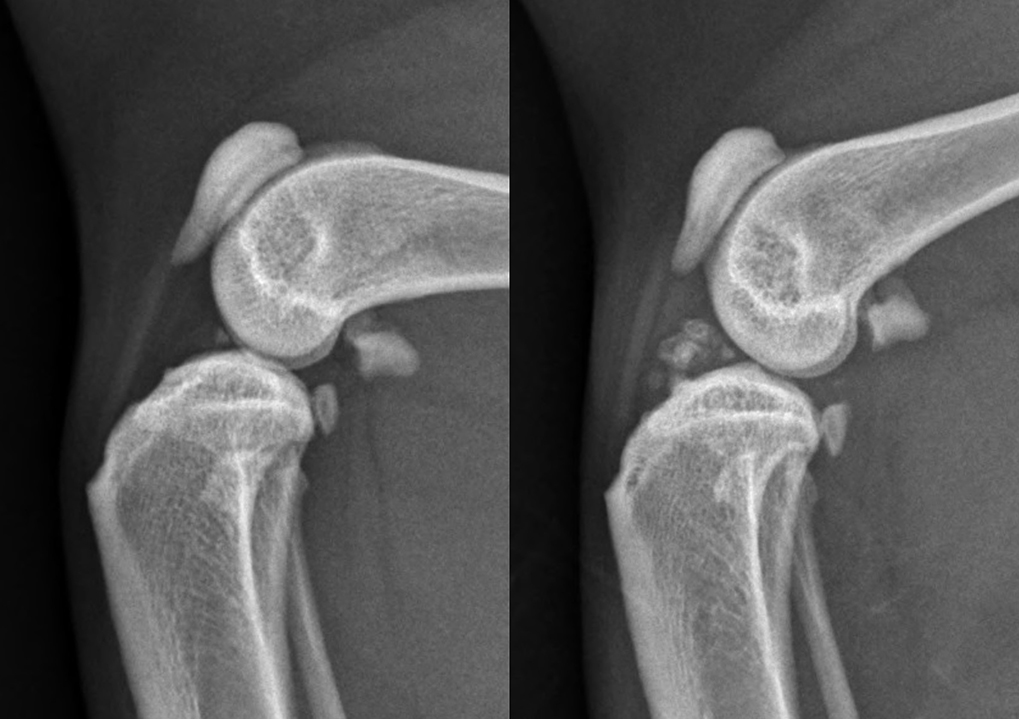
So, if the mineralisations are small, care must be taken not to over-interpret this finding; if it is not associated with joint effusion, lameness and pain it is likely incidental. However, if those mineralisations are larger, they are more likely to be associated with degenerative changes.
Radiographs of cats with CrCL rupture typically show joint effusion, although this can be more difficult to appreciate in cats. Cranial displacement of the tibia may be apparent and, in degenerative or chronic cases, changes associated with OA may be evident. Half of the cats in one study showed distal displacement of the popliteal sesamoid bone on lateral radiographs (Harasen, 2005) and this has also been proposed as a diagnostic marker for CrCL rupture in dogs (Figure 2).
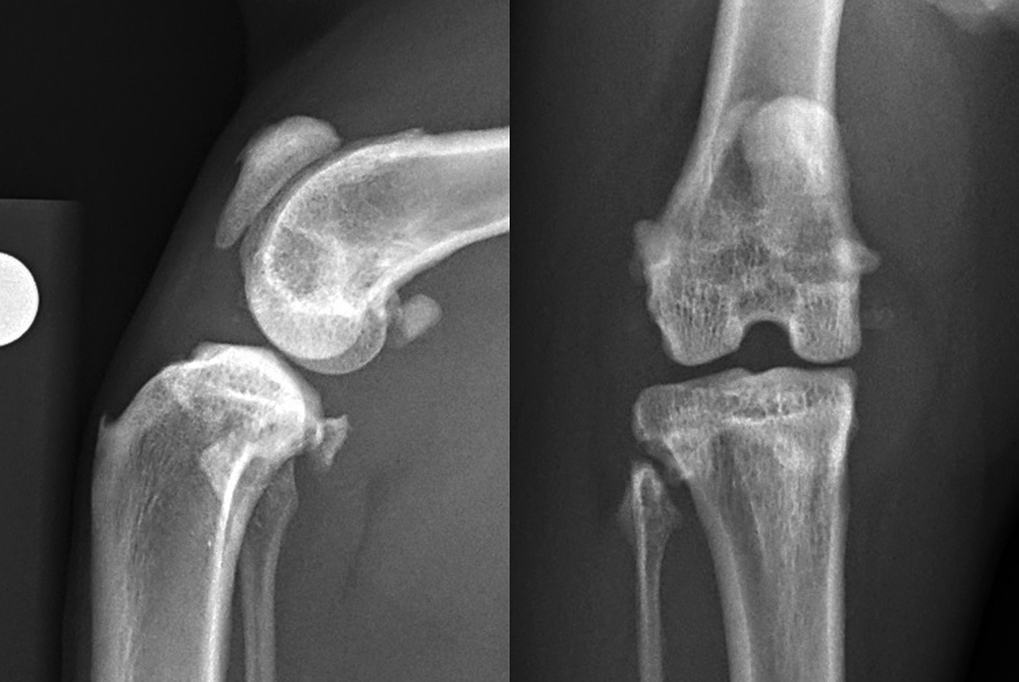
When we consider treatment options for CrCL in cats, non-surgical management has been shown to deliver satisfactory results in two small case series and is commonly trialled in this species. Scavelli and Schrader (1987) reported a case series of 18 cats with isolated CrCL rupture that were treated without surgery. Owners were advised to restrict their activity and to reduce weight in obese cats. Likely because the study was performed in 1987, no mention of medical management for these cases was reported. Mean age was 7.4 years and mean bodyweight 5.9kg. Radiography was not routinely performed prior to non-surgical management. Follow-up was performed at an average of 20.5 months post-diagnosis. Owners reported that lameness had resolved in all cases, with the mean duration of lameness being 4.8 weeks (range 1-16 weeks).
However, 14 cats had persistent instability in cranial drawer. Radiographs were performed in 14 cats at follow-up and in 12 of these showed periarticular osteophytosis (7) and intra-articular mineralisation (12). The authors of this study proposed that small-frame animals may be able to shift their weight more easily to the unaffected limb and, thereby, rest the affected limb, and that the cat’s agility may give it an added advantage in this regard, explaining their positive results with non-surgical management. They also argued that the relative ease of restricting a cat’s activity made it an ideal candidate for non-surgical management – something that I am not sure I entirely agree with from an owner’s perspective.
A study published more recently in 2022 evaluated a very similar cohort of cats to the previous study in terms of age and weight (Stoneburner et al, 2022). Of the 18 cats in this study, 17 were prescribed oral analgesics. Additional recommendations included exercise restriction for a mean of 6.7 weeks, treatment with polysulfated glycosaminoglycans or glucosamine and chondroitin supplements and weight loss. While this study had a longer-term follow-up at a mean of 66.5 months, it has some significant limitations in that the cases were only evaluated via phone or email correspondence via a questionnaire, so this study does not really help us assess the stability of these joints long-term. Thirteen of these cats became weight bearing within a week, four became weight bearing between two to four weeks and one took more than a month to start weight bearing. Fifteen of the 18 cats had owner-perceived clinically normal mobility with no observable lameness within three months after medical management was initiated. One cat took more than three months to become normal and two never returned to normal mobility. When the Feline Musculoskeletal Pain Index (FMPI) scores were evaluated, only two cats had scores more than 1 (with this questionnaire, scores from 0-4 were assigned, with 0 representing the cat being least affected and 4 representing the cat being most affected). One of these cats was obese and the other had bilateral CrCL rupture.
Surgical intervention for CrCL rupture has been proposed to produce a quicker and more durable recovery in cats over medical management (Harasen, 2005; Scavelli and Schrader, 1987; Umphlet, 1993). It has also been postulated that surgical intervention might lead to decreased development of OA. However, while we know that OA progresses following CrCL transection, no study has compared development between non-surgical and surgically treated cats, so the theory that OA progression will be delayed in a surgically stabilised stifle remains unproven.
Even strong advocates for non-surgical management tend to recommend surgical intervention in cats where lameness has failed to resolve within four to five weeks. Obesity has been considered an indication for surgical management by some authors, due to the presumed decreased ability to shift weight and rest the injured limb. Similarly, cats with bilateral stifle instability have been reported to have a longer recovery period with non-surgical management and, therefore, may justify earlier surgical intervention.
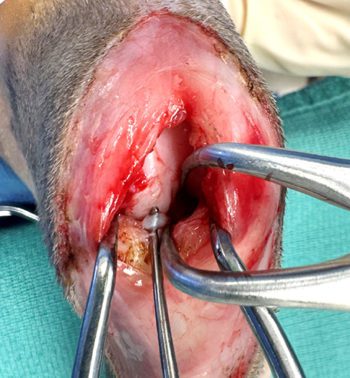
Meniscal damage occurs in up to 67% of cats with CrCL rupture (Ruthrauff et al, 2011); therefore, surgery for meniscal assessment and concurrent stifle stabilisation has been recommended (Figure 3). While, as was mentioned previously, the presence of meniscal calcification alone is not necessarily an indication for surgical intervention, as cats with larger mineralisations show more signs of joint degeneration, including specifically, degenerative changes in the CrCL, such cats may be more likely to benefit from surgical exploration.
The most commonly used technique in cats is static stabilisation with a lateral suture (De Sousa et al, 2014; De Sousa et al, 2015). This technique is usually favoured due to the simplicity, low cost and reduced invasiveness when compared with tibial osteotomies and intracapsular stabilisations. Additionally, a biomechanical study that compared the under-and-over intracapsular technique to the standard fabello-tibial suture demonstrated that a 40lb monofilament nylon prosthesis, tightened at 20N, produced more favourable stabilisation of craniocaudal cruciate-related stifle instability than a 0.5cm wide strip of fascia lata applied intra-capsularly (Kneifel et al, 2018).
A study by Harasen (2005) detailed eight cats with isolated CrCL injury. The mean age was 8.5 years and the mean weight 6.5kg. All eight cases were treated surgically with a lateral arthrotomy and then a lateral fabellotibial extra-capsular suture technique using monofilament nylon. Follow-up times in this study ranged from two months to six years postoperatively, and owners were asked to rate the function of the operated limb on a 0-5 scale with 0 being non-weight bearing at all times and 5 being completely normal use of the limb. All owners rated function of the limb at a 4 or 5. Two cats were noted to have slight stiffness on rising.
Normal use of the limb was reported to return from between two days to five weeks postoperatively, with a mean of just longer than two weeks. Based on this, the recovery period following surgery was postulated to be at least comparable, and potentially shorter than with non-surgical management. Unfortunately, due to follow-up being carried out over the phone with owners, the degree of stifle instability present in these cats and the progression of radiographic OA is not known, and cannot be compared with the results of non-surgical management.
To improve the lateral fabello-tibial suture technique in dogs, several modifications have been made since the original technique description, including the type of suture material used, knotting pattern and attachment points. A study by De Sousa et al (2014) aimed to establish quasi-isometric points for the technique of lateral suture placement and showed that the paired attachment points of the lateral fabella and a point 4mm proximal to the insertion of the patellar ligament provided the best quasi-isometric points for placement of a lateral suture in cats. While many factors exist that will influence the likelihood of a successful cruciate stabilisation, it seems reasonable that an implant that remains under relatively constant tension regardless of stifle position will probably perform best; therefore, these attachment points may be preferable.
However, in my experience and that of other surgeons, the femoro-fabellar ligament is relatively mobile in the cat compared to the dog and, therefore, often does not provide a firm anchor point. The use of bone anchors has been proposed to resolve this problem.
A biomechanical study (De Sousa et al, 2015) showed that suture anchors placed in the caudal aspect of the lateral femoral condyle are comparable to using the femoro-fabellar ligament as an anchor point, and this has become my preference due to concerns about how firm an anchor point the lateral fabella is in this species (Figure 4).
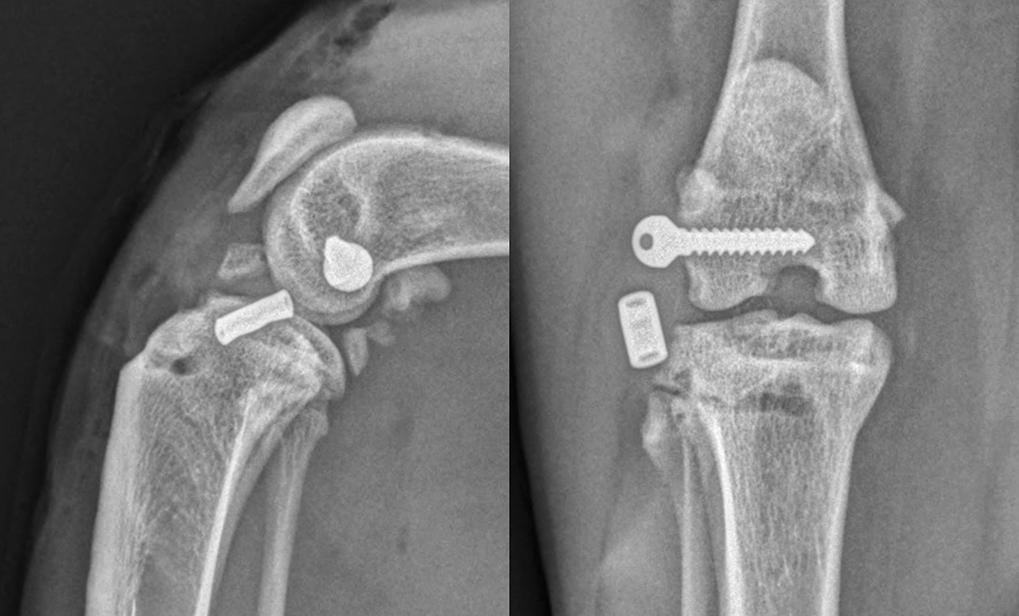
A retrospective cohort study contacted cat owners a mean of 41 months following a diagnosis of CrCL injury and compared outcomes between cats treated surgically with a lateral suture, and cats treated non-surgically (Boge et al, 2020). This study found that non-surgically treated cats had a lower FMPI score over those treated with a lateral fabello-tibial suture, indicating less chronic pain. In addition, the postoperative complication rate in this study was very high at 27.3%. The authors did state that this is an uncommon surgery for them to perform and, as such, these results could represent a learning curve.
The exact details of the surgical technique are also not available in the study, so whether bone anchors were used or not remains unknown. While limitations are associated with this study, it does prompt concern regarding the high complication rate and potentially suboptimal outcomes in some cats following lateral suture placement. As such, exploration of other stabilisation methods may be warranted.
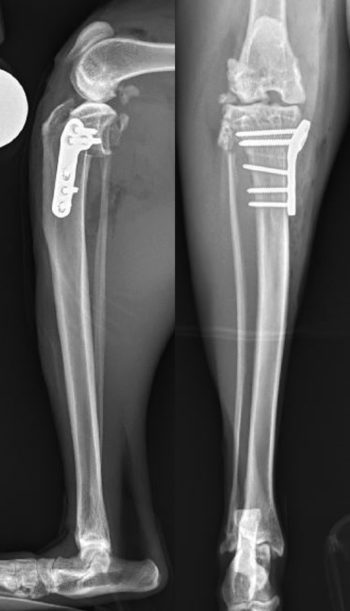
Techniques aimed at achieving dynamic stabilisation by modifying femoro-tibial biomechanics in dogs have been suggested to potentially offer more favourable functional outcomes than techniques providing passive stability. An increased tibial plateau angle has been described as a risk factor for isolated CrCL rupture in cats (Schnabl et al, 2009) and tibial plateau levelling osteotomy (TPLO) has been described as a potential treatment option (Figure 5).
Matis et al (2010) were the first to report their experience with TPLO in cats. Twenty-nine TPLOs were performed in 28 cats, with follow-up being achieved in 22 cases. No arthrotomy or meniscal treatment was performed in these cases. A 12mm biradial saw was used for the osteotomy and mini T-plates or 2mm TPLO plates were used for stabilisation. In four cats, a lateral suture was also performed because the stability achieved by the TPLO was not felt to be adequate. Complications were encountered in three cats, all of which were attributed to technical errors, and two other cats had a suboptimal outcome due to a failure to recognise caudal cruciate ligament rupture at the time of TPLO, which led to ongoing stifle instability. Six weeks postoperatively, the majority of cats still manifested lameness and muscle atrophy. However, owners reported that, after a short while, cats were able to return to jumping and normal activity. The authors reported that TPLO in cats was technically more demanding than in dogs due to the relatively small anatomy and brittle bones of cats.
Mindner et al (2016) also reported their experience with TPLO in 11 cats. In this case series, joint exploration and meniscal treatment as necessary were achieved via either arthroscopy or arthrotomy prior to TPLO, with partial meniscectomy being performed if meniscal pathology was detected. A 12mm saw blade was used for every cat and 2mm or 2.4mm locking TPLO plates were used for stabilisation. Following surgery, all stifles were stable in cranial tibial thrust. Follow-up was achieved in all cases to 12 weeks and by 4-12 weeks postoperatively, lameness had either resolved or was graded as mild in all cats. Owners reported a high level of comfort and mobility in all cats at final follow-up, although, a positive “sit test” was reported in seven cats. Minor intraoperative complications occurred in five cats and minor postoperative complications occurred in three cats, but no major complications were noted. The preliminary results from both of these studies support the use of TPLO in cats, but further studies are required to compare clinical results following TPLO and other methods before conclusions can be made regarding whether one technique offers superiority.
In the dog, dynamic stabilisation of the CrCL deficient stifle with tibial tuberosity advancement (TTA) is a popular technique and is supported by ex vivo mechanical studies. TTA is technically viable and has been reported in two cats (Perry and Fitzpatrick, 2010). Medial arthrotomies were performed for joint exploration in both cases, with no meniscal treatment being required. Two-prong tension band plates were used in both cases, with a 6mm cage used in one and a 3mm cage in the other. One major postoperative complication was reported where the screw in the cranial ear of the cage loosened and the tibial tuberosity proximal to the screw fractured. The screw was removed through a stab incision and no further treatment was deemed necessary. Both patients were weight bearing the day after surgery, and follow-up at 9 months in one cat and 15 months in the other revealed no lameness and maintained stifle stability in cranial tibial thrust. The only adverse observation was a positive sit test in one cat.
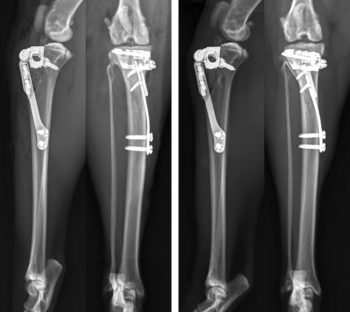
One attractive aspect of TTA is the ability to adapt the technique to treat cases of CrCL rupture with concomitant medial patellar luxation. I have used this technique in three cats (four stifles) which were diagnosed with varying grades of medial patellar luxation and CrCL (Bula and Perry, 2021). Stifle exploration via lateral arthrotomy revealed medial meniscal injury in all cases. Medial meniscectomy, partial parasagittal patellectomy, femoral trochleoplasty and tibial tuberosity transposition advancement using a 6mm cage, two-fork plate and 4mm spacer were performed in all four stifles (Figure 6).
By the two-week recheck, lameness was minimal and stifles were stable. Radiographic follow-up at eight weeks showed appropriate progression of osseous union in all cases. One cat experienced a major complication, suffering tibial fracture following a lapse in exercise restriction, and revision surgery was performed successfully with subsequent osseous union of both the fracture and the osteotomy site. Long term, with at least 12-month follow-up, all cats had return of previous level of function as assessed by both owner questionnaire using the FMPI and clinical evaluation. Based on our early experience with this technique, it does appear to warrant consideration in cats suffering from both conditions.
In summary, non-surgical management remains an option for treatment of CrCL insufficiency in the cat and appears to lead to resolution of subjectively perceived lameness in many cases within four to five weeks. However, it should be remembered that this is based upon relatively small case numbers, with a total of 16 cats between two studies, and largely subjective outcome measures. Surgical intervention should be considered in cases where lameness does not subside within this time frame and may also be used electively prior to this if a more rapid and durable response is desired, or if the cat is very active, obese, suffers from bilateral disease or is suspected to have meniscal injury.
No studies exist comparing different surgical treatments and, as such, choice of surgical technique remains at surgeon discretion. All surgical options appear to offer acceptable complication rates and a reliable resolution of lameness by 6-12 weeks postoperatively, and dynamic stabilisation techniques – particularly TPLO – appear to be gaining popularity.
Due to the low numbers of all surgical techniques reported, these necessarily have to be considered preliminary results, and also, likely represent the learning curve of all surgeons using these techniques.
Hip OA is relatively common in cats (Clarke et al, 2005), but is often not recognised, either because cat owners do not appreciate the pelvic limb lameness or because cats are better able to compensate for the resulting functional impairment (Witte et al, 2010).
The reported overall incidence of hip dysplasia in cats varies significantly between 6.6% in one study of 684 cats (Keller et al, 1999) and 32% in another study of 78 cats (Langenbach et al, 1998). The incidence is breed dependent, with purebred cats having a greater incidence (12.3%) than domestic shorthair cats (5.8%; Keller et al, 1999; Langenbach et al, 1998; Peiffer et al, 1974; Root et al, 1987). Of the purebred cats, the Maine coon is the most likely to be affected, with 18% to 21% of 284 cats reported positive based on standard Orthopaedic Foundation for Animals hip radiography (Keller et al, 1999). The Persian and Himalayan breeds are also over-represented (Keller et al, 1999).
Clinical signs associated with hip dysplasia include inactivity (Grierson, 2012), pelvic limb lameness worsened by exercise and climbing (Patsikas et al, 1998), difficulty or reluctance jumping (Patsikas et al, 1998; Grierson, 2012), inability or reluctance to climb stairs (Grierson, 2012; Holt, 1978), walking in a crouched position (Holt, 1978), howling while resting (Holt, 1978) and reluctance to squat to defecate (Holt, 1978). Cats with hip OA have also been shown to be less active at night when compared to normal cats using accelerometers (Guillot et al, 2012).
Lameness associated with hip dysplasia in cats can vary from relatively mild to severe with an inability to walk on the pelvic limbs (Patsikas et al, 1998). As hip dysplasia is commonly bilateral, the lameness can be relatively symmetrical, making it difficult to recognise. In these cases, the gait is simply stiff and stilted with shortened strides bilaterally. With unilateral problems, cats may unload a painful limb, even at rest (Kerwin, 2012). Cats with a unilateral pelvic limb lameness may demonstrate a hip hike, where the hip is elevated when the painful limb strikes the ground, and the tail may also be used asymmetrically to shift weight toward the more normal side when the cat is in motion.
The most commonly reported physical examination findings in cats with hip dysplasia are pain and crepitus upon extension of the hips, and muscle atrophy (Patsikas et al, 1998; Grierson, 2012; Holt, 1978). A reduced ROM is associated with hip dysplasia (Grierson, 2012). When examining the hip, ROM in abduction should be performed in addition to flexion, extension and rotation. Most cats can easily obtain 90° of pain-free abduction, similar to dogs (Kerwin, 2012). Cats with hip OA generally resent hip abduction, sometimes more so than flexion and extension (Kerwin 2012). As mentioned previously, abduction can also be used to evaluate the possibility of false-positive pain responses.
So, hip dysplasia and associated OA would explain Sushi’s lameness, the reduced jumping, the muscle atrophy and the pain response upon left coxofemoral joint extension. He is also a breed that is predisposed to developing hip dysplasia, so the signalment is appropriate. However, what could the pain response upon full extension of the left stifle be attributed to? One thing to be aware of is that while in cats with joint pathology pain is most frequently found on extremes of joint motion, this can be difficult to assess, as it is not uncommon for cats to resent this manipulation, even if the joints are normal and pain free. It could be that for Sushi, the inconsistent response was more resentment than true pain.
To further evaluate a suspicion of hip dysplasia and associated OA, our next step would likely involve orthogonal pelvic radiographs. Something to be aware of is that in cats, the normal acetabulum is generally shallower than in the dog (Keller et al, 1999). In dogs, one of the generally accepted criteria for normal acetabular depth is coverage of 50% or more of the femoral head. If this criterion were applied to cats then hip dysplasia would be overdiagnosed (Keller et al, 1999). Numerous cats of advanced age have less than 50% acetabular coverage of the femoral head, but no evidence of OA.
The most common radiographic finding in cats with hip dysplasia is a shallow acetabulum; sometimes, this is the only abnormality. Subluxation of the femoral head is not as common in cats as in dogs, but it can occur. Degenerative changes occur later and are less marked than in the dog. Early degenerative changes are noted primarily affecting the craniodorsal acetabular margins, with a low incidence of degenerative changes affecting the femoral head and neck (Figure 7).
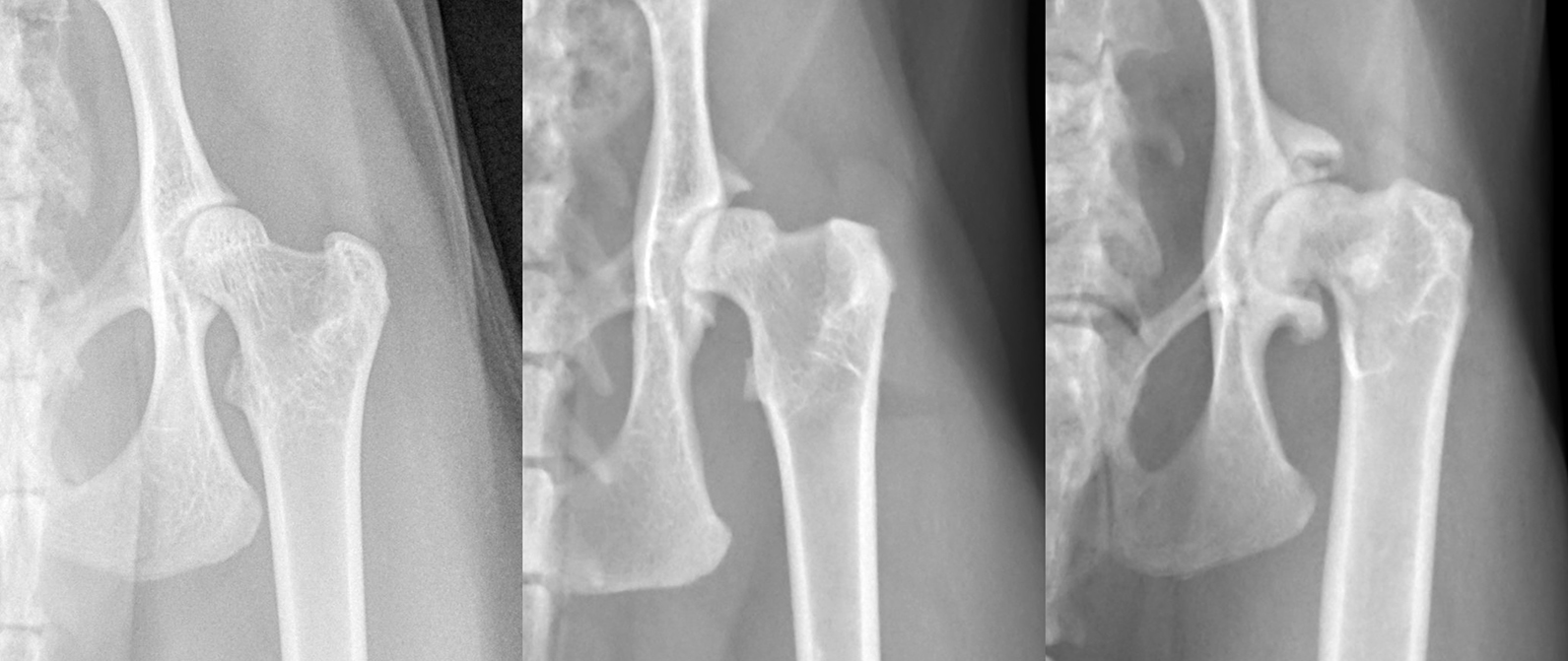
When remodeling of the head and neck does occur, it can present as deformation of the femoral head, mild femoral head flattening or poor demarcation of femoral neck. Exostoses can also be seen on the craniolateral edge of the femoral heads, and fine linear spurs may develop on the lateral aspect of the femoral necks. In chronic cases, the craniodorsal acetabular edge can become markedly deformed, extending caudally and laterally, and forming a new dorsal acetabular edge covering the femoral head, but early cases can be very subtle and easily missed.
Non-surgical management of feline hip dysplasia and associated OA hinges on a multimodal management plan tailored to the individual patient, but likely including components of environmental modulation, activity moderation, physical rehabilitation, dietary modification, weight management, NSAIDs and adjunctive analgesics as necessary. Where non-surgical management is ineffective, or in cases where this is anticipated to be required lifelong and an alternative option is desired, two recommended options for surgical management exist: femoral head and neck excision (FHNE), and total hip replacement (THR), with either cemented or cementless acetabular components available. FHNE is a salvage procedure intended to alleviate pain associated with movement of diseased or injured coxofemoral joints. The clinical results reported following this procedure vary significantly depending on the individual paper and the outcome measures used. In one study (Brezon et al, 1980) based on owner perception assessed by questionnaire postoperatively, seven cats had FHNE performed and excellent limb usage was reported in all, but in another paper, only 38% of small dogs and cats had a good outcome, with 20% having a satisfactory outcome and 42% of them being graded as poor (Off and Matis, 2010).
With functional results only being graded as good in 38% of cases, when recommending FHNE to pet owners, it should be emphasised that surgical outcome can be unpredictable, regardless of the weight of the animal.
It is also important for owners to be aware that, following FHNE, high levels of postoperative rehabilitation are often required to achieve optimal long-term function (Davidson et al, 2005). Successful outcome after FHNE is dependent on sufficient periarticular muscle competency for maintenance of a functional and durable pseudoarthrosis; as such, if owners cannot commit to physical rehabilitation, or perceive that their cat will not be tolerant of a postoperative rehabilitation programme, alternative treatment options should be considered in preference.
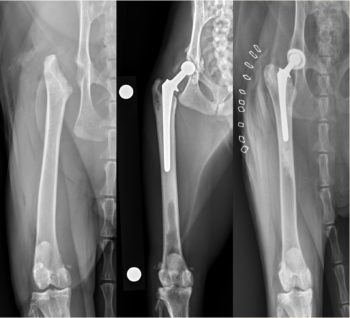
Although FHNE has the potential to return some dogs and cats to nearly normal function, inconsistent clinical results have fuelled a trend toward the preferential choice of THR as the standard of care salvage surgery (Figure 8).
The rationale behind THR in cats includes the desire to improve quality of life while maintaining normal biomechanical function in cats that are affected by coxofemoral pain and/or dysfunction (Liska, 2010). This is undoubtedly beneficial when considering salvage procedures of the coxofemoral joint, as restoration of normal hip joint function is certainly the preferred outcome. Although no objective data comparing functional outcome in cats after FHNE and THR have been reported, it seems likely that cats will benefit from joint replacement in preference to FHNE (Witte et al, 2010).
A recent report (Rodiño Tilve et al, 2022) described the outcome following 56 total hip replacements in 44 cats. Median age was two years, and median bodyweight 5kg. The British shorthair and domestic shorthair were the breeds most commonly undergoing the procedure, and most cats were neutered males. During a median duration of follow-up of slightly longer than two years, nine major complications and two minor complications were reported. The FMPI score significantly improved postoperatively and owner satisfaction was reported as “very good” in more than 90% of cases.
While fractures of the femoral head and neck can be associated with trauma, slipped capital femoral epiphysis (SCFE), or spontaneous femoral capital physeal fracture, is a unilateral or bilateral progressive displacement of the capital femoral epiphysis from the proximal femoral metaphysis through the growth plate, without a history of significant trauma, with various factors including genetics, obesity, endocrine imbalances, neutering and sex believed to play a role in the development of the disease. The condition normally affects cats between one to two years of age, most often in neutered males, and often in those with an increased body condition score.
Breed predispositions have also been reported, with the Maine coon being 12 times more likely to develop this condition than the overall population of cats (Borak et al, 2017). The median age of Maine coons presenting with this condition is 21 months (Borak et al, 2017).
Cats with SCFE will typically present with either acute or chronic, moderate to high grade pelvic limb lameness, weakness, pain and reluctance to jump. Studies have reported that hindlimb lameness, pain on femoral abduction and pain on full extension of the hip are almost universally encountered with this condition. Additionally, this condition is commonly bilateral; in fact, in the Maine coon, a study reported that 41% of cats (7 out of 17) were bilaterally affected (Borak et al, 2017).
So, a SCFE would explain Sushi’s lameness, the reduced inclination to jump, the muscle atrophy and the pain response upon left coxofemoral joint extension. The Maine coon is also predisposed to this condition, and Sushi ticks multiple other boxes in terms of the typical signalment being male neutered, and at an age that is close to the median reported for Maine coons specifically. While he was not overweight, the Maine coon is obviously a larger breed and males do gain higher bodyweights than females. A study evaluating this condition in the Maine coon specifically postulated that obesity may not contribute to SCFE in the breed, but the comparatively high bodyweight and associated mechanical overload could promote the development of SCFE (Borak et al, 2017).
To evaluate further for a potential diagnosis of SCFE, orthogonal radiographs of the pelvis and femora would likely be performed. For most cases of SCFE, radiography will reveal displacement of the femoral epiphysis relative to the metaphysis on one view or another (Voss et al, 2009a). In some cases, this is very clear, while in others, only a very small step or mild widening of the physis is evident, which can be more challenging to recognise. Osteolytic and sclerotic changes become evident in the metaphyseal bone along with femoral neck resorption with time (Forrest et al, 1999; McNicholas et al, 2002).
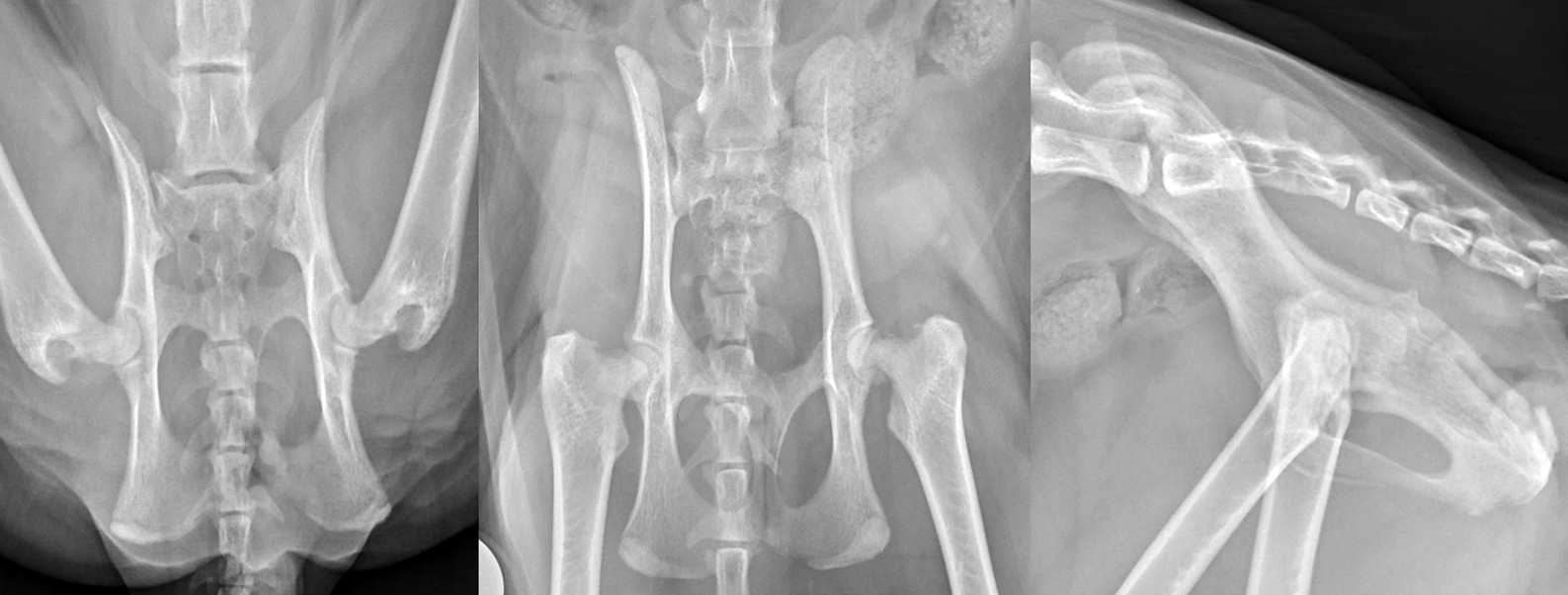
Something important to note regarding radiographs of cats with SCFE is that the radiographs are very position dependent. When the displacement is mild, this may only be evident on one view. This is particularly true in early cases where only mild physeal widening is apparent, so it is important to always take two ventrodorsal views, including both a frog-legged view and an extended leg view, to give yourself the best chance of visualising the problem. The frog-legged view depicts the hips with less tension on the joint capsule and, typically, will be the best view to allow confirmation of mild metaphyseal separation (Lafuente, 2011). I also find an open-leg lateral view can be very helpful to visualise the caudal displacement of the epiphyseal segment (Figure 9).
If a diagnosis of SCFE is confirmed, treatment options will be dependent upon the chronicity of the condition. Non-surgical treatment invariably results in formation of a hypertrophic pseudoarthrosis (Bennett, 1975; Pérez-Aparicio and Fjeld, 1993), which is associated with poor clinical outcomes and is not recommended. In acutely affected cats, without evidence of metaphyseal bone resorption and lysis, reduction of the fracture and stabilisation using parallel Kirschner wires or a screw is recommended. For chronic fractures or those with radiographically evident metaphyseal resorption, decision making is more complex, but it is likely that remodelling of the fracture ends will preclude accurate apposition; as such, FHNE or THR are generally recommended (Voss et al, 2009b).
For Sushi, a sedated orthopaedic examination revealed mild crepitus upon manipulation of the left coxofemoral joint. Due to the presence of crepitus, and the fact that SCFE remained on the differential list, an Ortolani test was not attempted.
No instability in cranial drawer or cranial tibial thrust was palpable within the left stifle, the patella was tracking normally and no stifle effusion was palpable. The remainder of the sedated orthopaedic examination was within normal limits. Radiographs of the pelvis and femora revealed a Salter-Harris type 1 fracture of the capital femoral physis, which reduced spontaneously when positioned for the craniocaudal view of the femur (Figure 10).
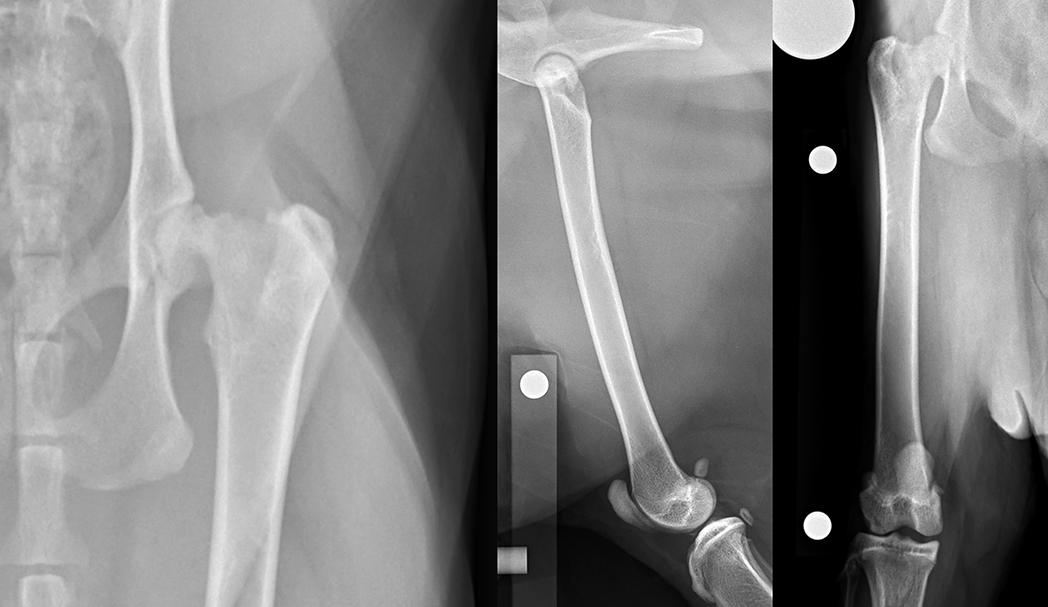
Due to the poor outcomes associated with non-surgical management, early surgical intervention was recommended. The repair of proximal femoral fractures, however, can be challenging because of several unique biologic and mechanical factors, including concurrent trauma to the fragile vascular network, eccentric loading of the femoral head and neck, and the limited bone stock available for stabilisation.
We know that complications increase in cases less than six months of age, in cases where anatomic or close to anatomic reduction is not achieved, in cases with ipsilateral hip injuries, in cases where surgery takes place later, in cases where damage has occurred to the blood supply and in cases where overly rigid fixation is used.
While the degree of damage to the blood supply for Sushi remains unknown, he is not subject to any of those other risk factors; anatomic reduction remains possible and lameness has only been apparent for three days. Additionally, no evidence existed of fracture site remodelling, metaphyseal resorption or sclerosis on the radiographs; as such, he presented as a reasonable candidate for primary surgical stabilisation.
Capital physeal fractures can be stabilised using either open or closed approaches. When we think about proximal femoral fractures, closed reduction, with the assistance of intraoperative fluoroscopy, has some potential benefits. It has the potential to limit iatrogenic trauma to the blood supply of the proximal femur, reduce the incidence of femoral neck resorption, which has been reported following open reduction and internal fixation, and to optimise the use of the limited bone stock available, so, this is what we elected to do for Sushi.
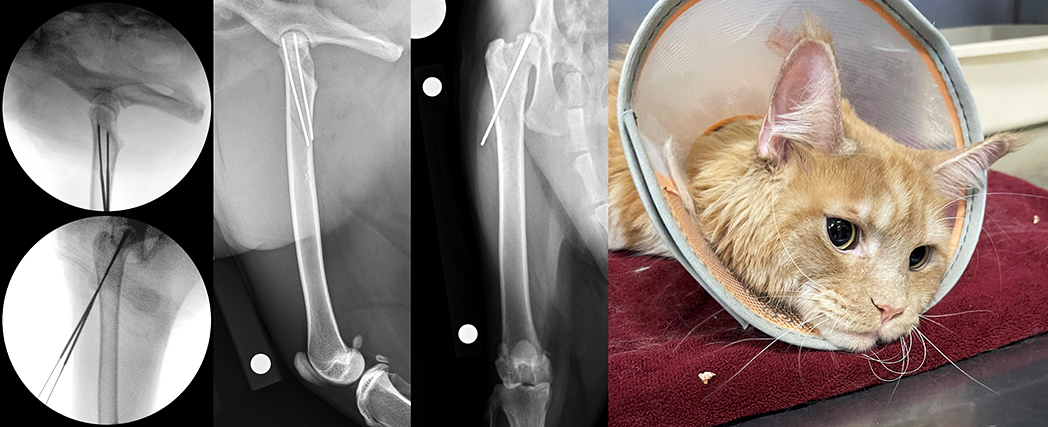
Fracture reduction was performed under fluoroscopic guidance. A 16G hypodermic needle was inserted, beginning at the junction of the proximal and middle thirds of the femur, such that it would enter the bone in the region of the third trochanter, and this was oriented to follow the angles of inclination and anteversion. A Kirschner wire was then passed through the hub of the needle and slowly advanced into the femoral neck, with pin orientation and positioning being verified via intraoperative fluoroscopy. Continued advancement was sequentially monitored until the pin was well seated within the proximal fragment, but was not penetrating the articular cartilage.
The procedure was then repeated to achieve two-pin fixation of the fracture, and implant positioning was checked on orthogonal views. Finally, the pins were cut a distance away from the lateral cortex to facilitate pin removal later. Postoperative radiographs confirmed satisfactory alignment, apposition and implant placement, and Sushi was discharged with instructions for strict rest for the following four weeks. At his four-week postoperative re-check, lameness had resolved, no pain or crepitus was reported upon left hip manipulation, and radiographs revealed appropriate progression of osseous union. The pins were removed through a single stab incision and a gradual return to normal activity was recommended.
It should be noted that the list of differential diagnoses addressed here is certainly not exhaustive. Another potential condition that would have been considered would be lumbosacral disease, which is likely under-recognised in cats. No breed predilection has been identified, but reports are sparse, with only five or six cases in each publication.
Recognised clinical signs include reluctance to jump, low tail carriage, elimination outside the litter box, reluctance to ambulate, pelvic-limb paresis, urinary incontinence, and constipation; on examination, cats generally manifest lumbosacral pain on palpation in addition to pain on full hip extension and abduction. So, significant overlap exists between the clinical signs associated with this condition and those reported in Sushi.
As treatment options would be significantly different for this condition, while not addressed in depth in this article, it remains an important differential to keep in consideration in cats with these clinical signs.