23 Jul 2024
Tyler Willard and Renata Stavinohova explain how to diagnose and treat this ocular canine ailment.

Image © Stephen Davies / Adobe Stock
The cornea is made up of four distinct layers: the epithelium, stroma, Descemet’s membrane and endothelium. It is richly innervated by the ophthalmic branch of the trigeminal nerve, and ulceration can be a source of pain and discomfort for patients.
The surface of the cornea is covered by the tear film, which lubricates, oxygenates and provides nutrition; disturbances can cause ocular discomfort and increase the risk of ulceration.
One aim of the canine cornea is to allow and refract light into the anterior chamber, through the lens and to the retina. This is achieved through its unique transparent properties and curvature, and as such, corneal ulcers and complications can be detrimental to patient vision quality.
The cornea also plays a part in maintaining appropriate pressures in the globe, and perforation of the cornea risks losing the affected eye.
Common causes of corneal ulceration include:
It is important to identify any contributing factors that may cause ulceration to guide your treatment plan. Diagnostic work-up of all patients should include (unless contraindicated) examination of the surface of the globe and the adnexa, Schirmer tear testing, tear film break-up time, fluorescein staining and examination of the bulbar aspect of the third eyelid after application of a topical local anaesthetic.
The examination should be performed grossly first to assess overall globe presentation and to look for corneal foreign bodies, or eyelid abnormalities (neoplasms, entropion or ectropion). Neuro-ophthalmic examination including palpebral and corneal reflex should also be performed (Figure 1).

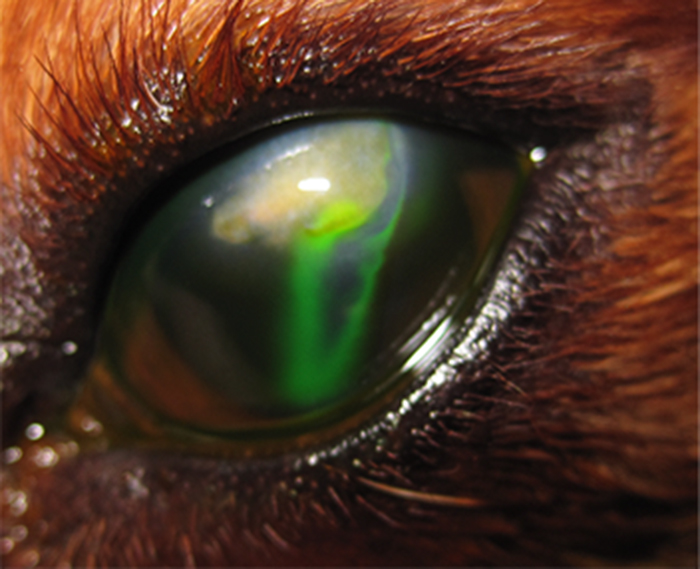
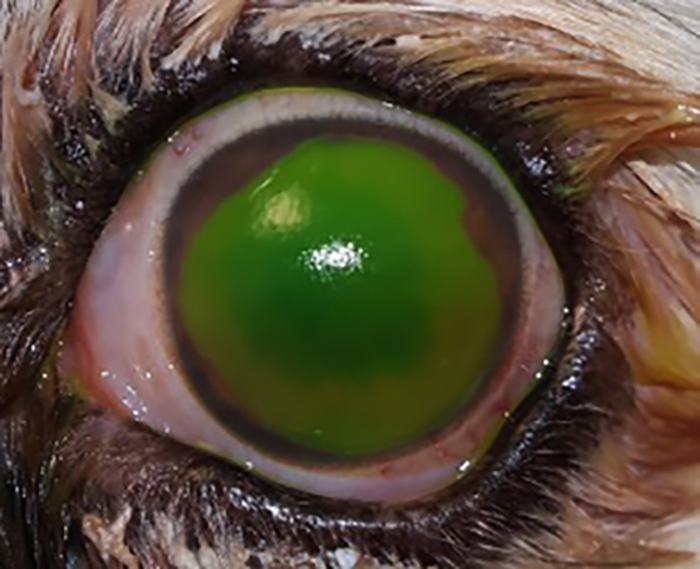
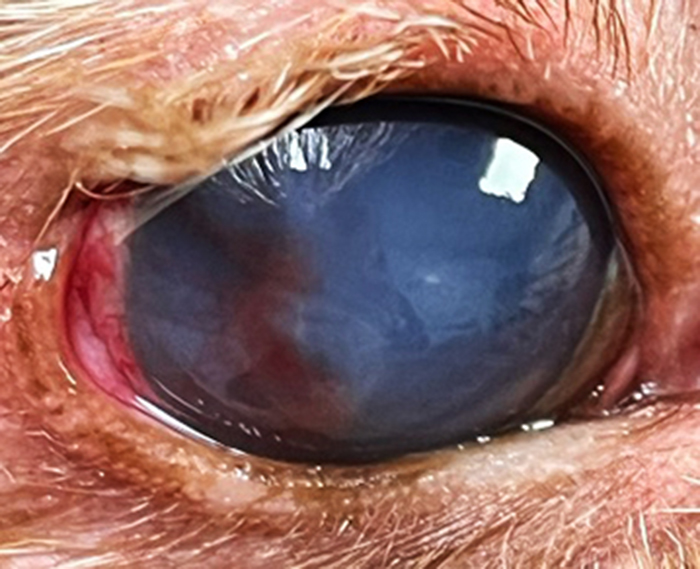
Figure 1. Corneal stromal ulcer in the right eye, associated with severe keratitis due to lagophthalmos (facial paralysis).
Figure 2. Positive Seidel test.
Figure 3. Superficial corneal ulcer in the left eye, associated with perilimbal vascularisation and conjunctivitis. Fluorescein stained. Macroblepharon can be appreciated.
Figure 4. Superficial corneal ulcer in the right eye, associated with corneal oedema, fibrosis and vascularisation.
Closer examination of the cornea and surrounding structures is best achieved with a slit lamp (a bio-microscope), but can be done with an ophthalmoscope (set at +20 dioptres) or magnifying loupes. Try to identify any corneal neovascularisation, which indicates chronicity, and see whether the vessels are long and branching, such as with superficial infiltration of vessels, or straight and non-branching, which indicates deeper corneal involvement.
It is important to look out for eyelash abnormalities, as these can be a causative factor or a reason for delayed ulcer healing:
The bulbar aspect of the third eyelid should be assessed for hidden foreign bodies and can be achieved using a topical local anaesthetic and Bennett Cilia forceps to gently manipulate the membrane. This step must be performed after a Schirmer tear test (STT), as placing eye drops before an STT will alter the results.
Schirmer tear testing should be performed on all uncomplicated superficial ulcers, and care should be taken if a deep/melting ulcer, descemetocele or perforated ulcer is suspected.
STT results should be more than 15mm in 60 seconds, and 15mm or less should prompt further investigations for dry eye. Eyes with corneal ulcers will always have higher STT values; therefore, it is important to perform STT in both eyes. Tear film break-up time should also be performed to determine tear quality. A drop of fluorescein stain is applied to the eye and the eyelids are closed and opened again to evenly distribute the stain. It is then examined under cobalt blue light and timed for when the stain starts to break apart. In a healthy canine patient, the break-up time can vary between approximately 6 to 29 seconds1,2.
Fluorescein is a water-soluble dye that helps to identify corneal ulcers by adhering to the stromal and basal layers of the damaged cornea, appears as a bright yellow/green stain on the defect and fluoresces under cobalt blue light.
It is important to rinse the dye from the surface of the eye (using sterile saline) after application, as it also has an affinity for cellular debris and mucus, which could result in a false-positive stain uptake.
Fluorescein stain is also useful in identifying perforations. When examined under cobalt blue light, a positive Seidel test identifies aqueous humour leaking from a full-thickness defect by diluting the dye, making it appear dark blue and leaking from the defect site (Figure 2).
Cytology and culture are useful tools in the management of corneal ulcers, and should be encouraged in any complicated or slow-healing ulcer.
Corneal cytology should ideally be performed by using a cytobrush to gently harvest surface corneal cells, which can then be transferred to a clean glass slide. Samples for culture and sensitivity can be taken using the appropriate swab and medium provided by the laboratory. A smaller sampling tip is preferred. Samples for PCR can also be taken with a cytobrush or sterile cotton tip, and should be transported in a plain tube unless an alternative medium has been suggested by the laboratory.
It is important to take great care when sampling for cytology or culture due to a weakened cornea being at greater risk of developing a perforation. Corneal cytology and culture allow for a better understanding of potential pathogen involvement, and will aid in the selection of appropriate antibiotics.
It is important to note that chloramphenicol is broad-spectrum, with activity versus Rickettsia, Chlamydophila and Mycoplasma species, as well as Gram-positive and Gram-negative agents, except for Pseudomonas aeruginosa. Staphylococcus and Streptococcus species isolates from dogs with bacterial keratitis were susceptible to chloramphenicol, whereas Pseudomonas species isolates were resistant3,4.
Therefore, in cases where finances are limited, the use of in-house cytology can still be beneficial in helping guide medical therapy.
Superficial erosions (Figures 3 and 4) affect the epithelial layer of the cornea. Depending on the extent, location and underlying cause, superficial ulcers are usually quick to heal with the correct identification and appropriate treatment. Diagnosis of a superficial ulcer usually appears as a well-defined area of fluorescein stain uptake on the cornea with no evidence of deeper stromal involvement.
Management of an uncomplicated superficial corneal ulcer should include a broad-spectrum topical antibiotic such as chloramphenicol, a topical cycloplegic to manage any reflex uveitis (atropine sulphate or cyclopentolate hydrochloride), adequate analgesia (systemic NSAIDs, paracetamol), lubrication with a hyaluronate-based lacrimomimetic and the use of an Elizabethan collar (E-collar) to minimise the risk of further trauma.
Cytology and culture should be performed if corneal cellular infiltration or purulent discharge is present.
These patients should be rechecked in five to seven days, and owners at home should look out for increased ocular discomfort, change to ocular discharge or the development of white/yellow/grey/green colour infiltrates in the cornea, which could be suggestive of deeper stromal infection – any of which would require prompt re-examination and adjustment of the treatment plan.

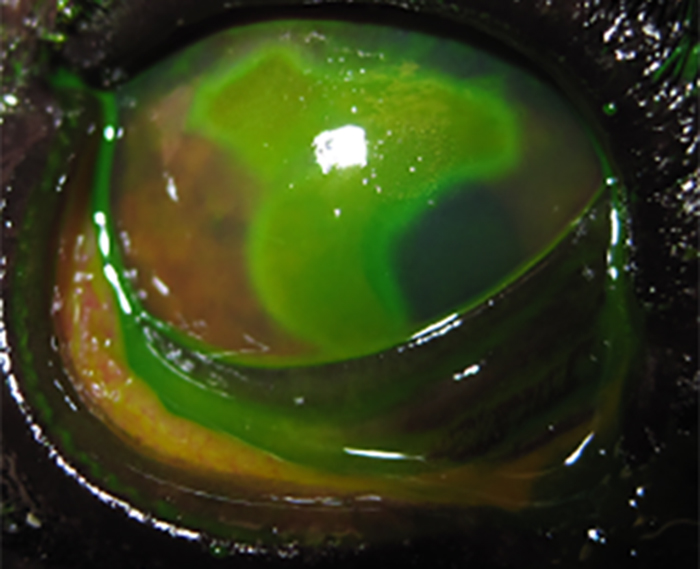
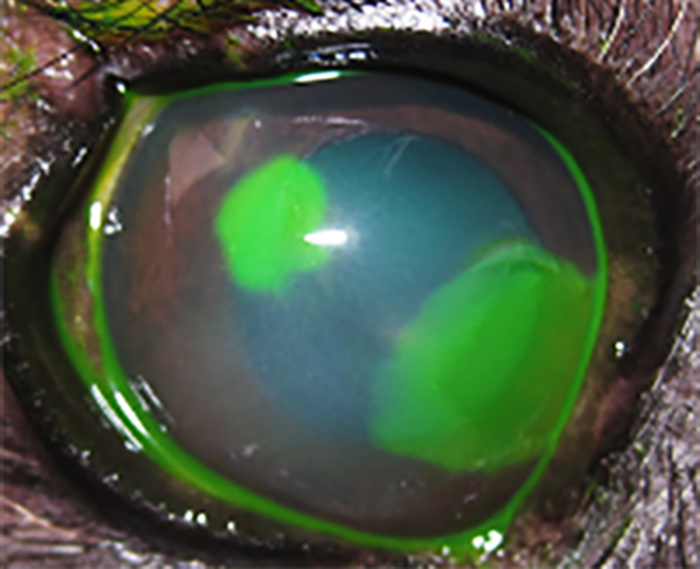
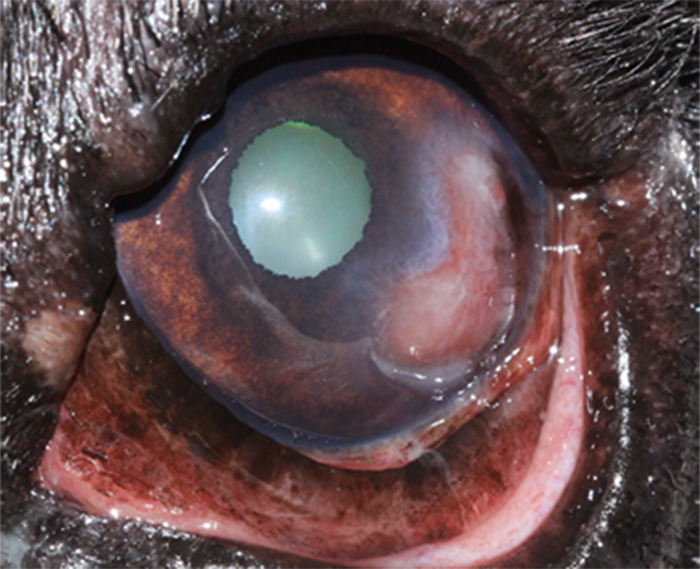
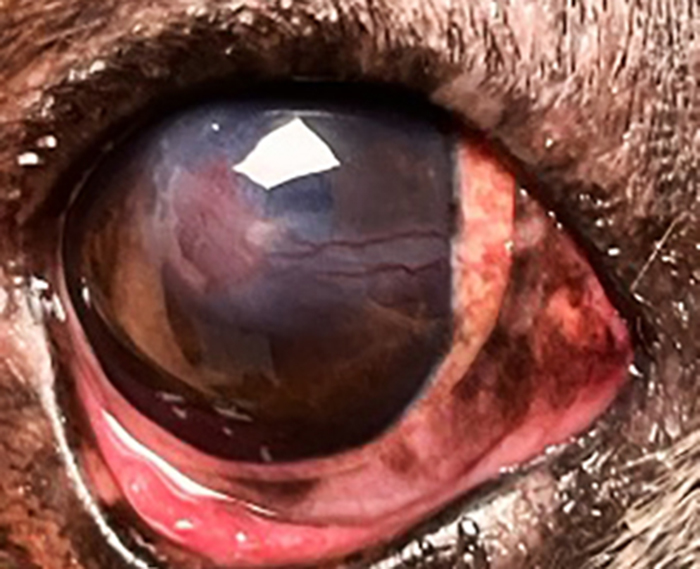
Figure 5. Spontaneous chronic corneal epithelial defect (SCCED) in the left eye, associated with reflex uveitis. Fluorescein stained.
Figure 6. SCCED in the right eye. Fluorescein stained.
Figure 7. SCCED in the right eye, associated with corneal oedema. Fluorescein stained.
Figure 8. SCCED in the left eye, associated with vascularisation, fibrosis and granulation tissue. Macroblepharon can be appreciated.
Figure 9. SCCED in the right eye, associated with a small corneal abscess (a white, central corneal opacity). Macroblepharon, conjunctival hyperaemia, corneal oedema, fibrosis and vascularisation can be appreciated.
SCCEDs (Figures 5 to 9) are a type of non-healing ulcer that can be found in dogs of any age or breed, but are over-represented in middle-aged dogs and breeds such as the boxer, Boston terrier, French bulldog and Labrador retriever5.
SCCEDs typically appear as a non-infected superficial ulcer with a surrounding non-adhering or loose sheet of epithelium (with or without corneal neovascularisation, which can present in more than half of cases).
Although the pathophysiology is not completely understood, it is postulated that the stromal hyaline acellular zone detected in SCCEDs results in a reduced ability to form normal adhesion complexes of the corneal epithelium to the basement membrane and stroma. Corneal cytology should be considered if corneal cellular infiltration or purulent discharge is present, and/or multiple antibiotic therapies were attempted.
Diagnosis is usually achieved by appearance (a loose border of non-adherent epithelium), fluorescein staining pattern (a diffuse halo of stain weakly under-runs the borders of the erosion) and a failure to heal over the expected period for a superficial ulcer.
It is important to rule out underlying pathology resulting in a non-healing superficial ulcer, and this is particularly important for young dogs where SCCEDs are under-represented. Investigations should include Schirmer tear testing to rule out underlying dry eye, fluorescein staining to evaluate ulcer appearance and tear film break-up time, examination of the bulbar surface of the third eyelid to rule out foreign bodies, and an examination of the palpebral margins and conjunctiva to rule out distichiasis, trichiasis or ectopic cilium, respectively.
Several treatment options are available for the management of SCCEDs, although these have varying degrees of resolution and some require referral to an ophthalmologist. Treatment options are briefly outlined in Table 1.
| Table 1. Treatment options for spontaneous chronic corneal epithelial defects | ||
|---|---|---|
| Treatment option | Procedure outline | Successful outcome after treatment |
| Cotton tip debridement | ● Topical local anaesthetic (proxymetacaine) ● Sterile cotton tip is used to debride the non-adherent epithelium ● Normal epithelium is strongly adhered to underlying stroma and should withstand debridement, so debridement should continue until all loose epithelium is removed |
● Approximately 50% after one treatment, average healing time 14 to 24 days ● Can be repeated6 |
| Diamond burr debridement (Figure 10) | ● Topical local anaesthetic or general anaesthetic ● Ocular surface preparation with 0.2% povidone-iodine (cornea) and 0.1% povidone-iodine (eyelids)** ● A sterile cotton tip is used to debride the non-adherent epithelium ● Use a handheld motorised diamond burr, 3.5mm medium grit ● Apply gentle pressure to the ulcer bed and move circularly for approximately one minute, or until loose epithelium is removed |
● Up to 73.9% to 87.5% after one treatment, average healing time in approximately 14 days ● Can be repeated7 |
| Anterior stromal puncture (note: consider referral to an ophthalmologist) | ● Topical local anaesthetic or general anaesthetic ● Ocular surface preparation** ● Epithelial debridement (cotton tip) ● Use 25G needle clasped in a haemostat so the needle tip is barely showing (alternatively, an insulin needle can be used) ● Make many small punctures 0.5mm to 1mm apart from the exposed stroma and extend this 1mm into healthy surrounding corneal epithelium |
● Up to 97.4% to 100% after one treatment, average healing time in 10 to 14 days6 |
| Grid keratotomy (note: consider referral to an ophthalmologist) | ● Topical local anaesthetic or general anaesthetic ● Ocular surface preparation* ● Epithelial debridement (cotton tip) ● Insulin needle is used to created corneal grids |
● Up to 83% success after one treatment, average healing time approximately 11 to 14 days8 |
| Superficial lamellar keratectomy/keratectomy (Figure 11; note: requires referral to an ophthalmologist with microsurgical skill and an operating microscope) |
● General anaesthetic
● Topical local anaesthetic ● Ocular surface preparation** ● Area surrounding non-adherent epithelium is outlined with a set depth restrictive corneal knife, and then undermined with a corneal lamellar crescent knife ● Free corneal tissue is then removed and attachments are trimmed as necessary |
● Up to 100% success rate after one treatment, average healing in 9 to 14 days ● Requires referral ● Requires general anaesthetic6 |
| Corneal thermal cautery (note: requires referral to an ophthalmologist who has experience with the technique) | ● Topical local anaesthetic ● Ocular surface preparation with 0.2% povidone-iodine ● Epithelial debridement (cotton tip/DBD) ● Corneal thermal cautery using a low temperature, handheld thermal cautery pen ● Aim to creature punctate superficial burns ● Include stromal bed of defect and extending 2mm past epithelial margin |
● Up to 65% to 100% after one treatment, average healing time in 14 to 21 days9 |
Post-procedural care should include placement of a bandage contact lens8,10 (unless contraindicated), mydriatics and cycloplegic, topical antibiotic therapy until the ulcer has healed and regular lubrication with a hyaluronate-based ocular drop. Some ophthalmologists may also start plasma or serum drops topically, either as prophylactic treatment for keratomalacia (especially in the brachycephalic dog) or as an additional supportive treatment. Systemic NSAIDs and paracetamol should also be prescribed unless contraindicated.
These patients should be sent home with an E-collar until clinical resolution. A recheck appointment should be booked for 12 to 14 days (5 to 7 if the patient is brachycephalic), and the owner should be monitoring for signs of ocular discomfort, cellular infiltration of the cornea (usually white/yellow/grey/green in colour and can be suggestive of a deeper stromal ulcer) – any of which would require prompt re-examination.
Stromal ulcers (Figures 12 to 14) affect the stromal layer of the cornea (further classified as anterior, mid or deep), and descemetoceles are deeper still.
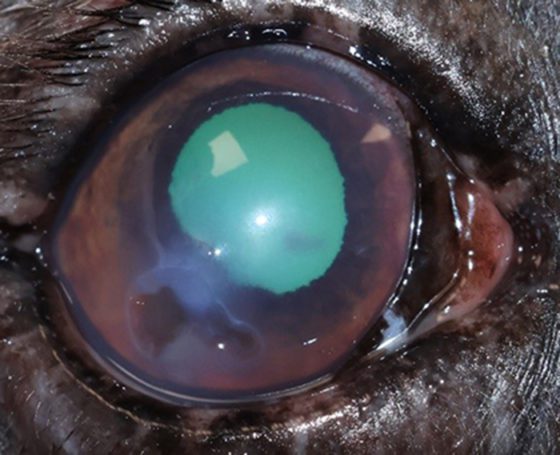
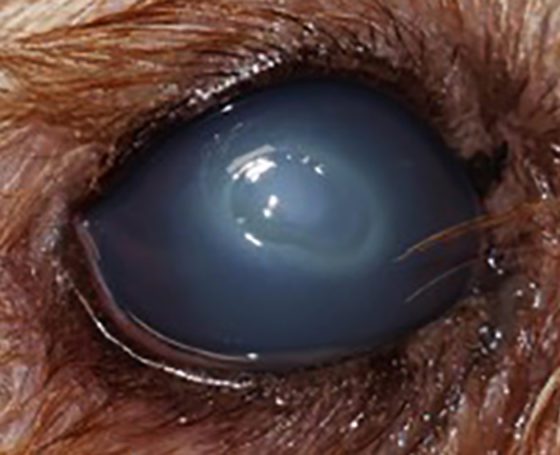

Figure 12. Deep stromal corneal ulcer, associated with cellular infiltration in the right eye, corneal oedema and reflex uveitis.
Figure 13. Deep stromal corneal ulcer, associated with cellular infiltration in the right eye, corneal oedema and reflex uveitis.
Figure 14. Deep stromal corneal ulcer associated with fibrosis and vascularisation in the right eye.
Where the defect is down to the Descemet’s membrane, the inner contents of the globe are held in place by just 10µm to 15µm of tissue, and as such, are at great risk of rupturing. These are ocular emergencies and require prompt treatment, and referral to an ophthalmologist.
Diagnosis can be achieved using the plan set previously described, and the slit beam feature of an ophthalmoscope can be used to gauge if the ulcer is deeper than the epithelial layer. A deep ulcer will have complete fluorescein stain uptake, while a descemetocele will not, as the Descemet’s membrane does not uptake stain.
A descemetocele (Figures 15 and 16)has a characteristic appearance often described as a doughnut or halo of stain uptake, with a dark unstained centre. Perforations can be identified through a positive Seidel test; however, some perforations can be plugged with fibrin or the iris itself.
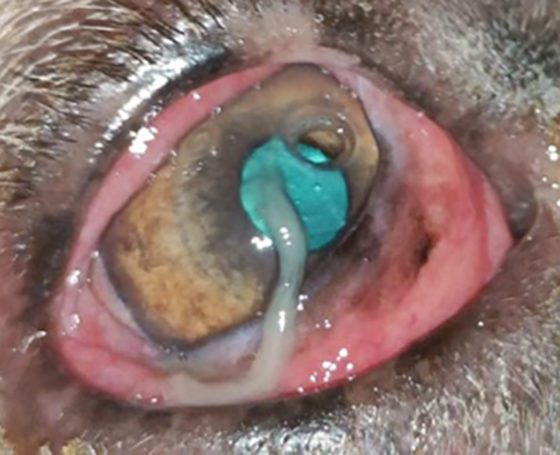
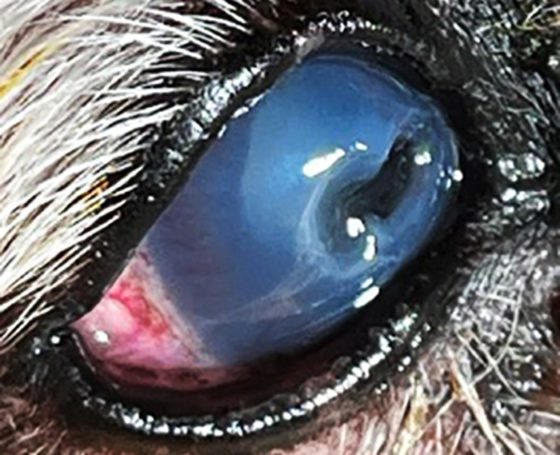
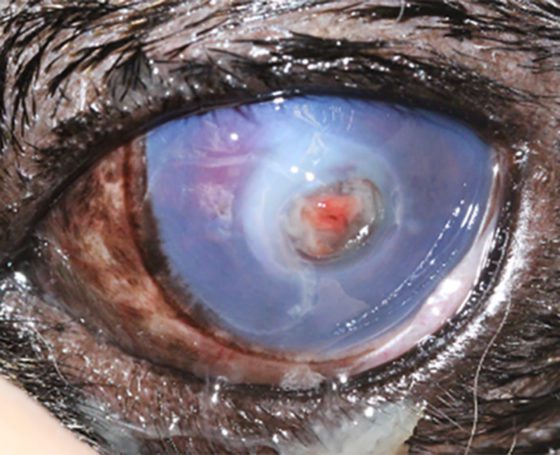
Figure 15. Descemetocoele in the right eye. Macrobleharon, conjunctivitis and mucopurulent discharge can be appreciated.
Figure 16. Descemetocoele in the right eye. Corneal oedema and perilimbal vascularisation can be appreciated.
Figure 17. Corneal perforated ulcer associated with iris prolapse and hyphaema in the left eye. Severe corneal oedema and fibrosis can be appreciated.
A perforated ulcer (Figure 17) with an iridial plug will have a pigmented centre, possibly within a fibrin plug. Hyphaema (blood in the anterior chamber) or hypopyon (white blood cells in the anterior chamber) can also be present.
Most of these eyes present with reflex uveitis and will be incredibly painful, often presenting with subsequent blepharospasm, discharge and hyperaemia.
It is important to make sure these patients have appropriate analgesia (systemic NSAIDs, paracetamol or opioids such as buprenorphine), as well as sedatives or anxiolytics (tramadol, acepromazine or gabapentin) if the patient is likely to cause further trauma.
Proper patient handling is key. An E-collar should be placed to minimise the risk of further trauma, any collars should be substituted for a harness to reduce pressure put on to the neck (which can increase intra-ocular pressure and risk of rupture) and handling should be kept to a minimum. Care should be taken when applying any eye drops, as should taking any samples for cytology or culture.
Patients with perforation, descemetoceles or deep ulcers approximately half the corneal depth and greater require surgical intervention, as well as regular monitoring. These patients are candidates for urgent referral to an ophthalmologist to determine if the eye is salvageable, the most appropriate surgical approach and to provide intense medical treatment.
Many surgical approaches exist for these ulcer types (for instance, corneo-limbal conjunctival transposition, corneal grafts, conjunctival flaps; Figures 18 to 20) and the choice of technique will depend on the location and depth of the ulcer, as well as the health of the surrounding corneal tissue. Enucleation is reserved for cases involving blind, painful eyes, extensive corneal defects/perforations and situations where the owner cannot afford corneal surgery.
Medical treatment consists of topical antibiotics as per the cytology and culture, plasma or serum, mydriatics and cycloplegic, lacrimomimetics (sodium hyaluronate) and amnion drops. If severe discharge is present, mucolytics, such as topical N-acetylcysteine, can also be added.
Keratomalacia (Figures 21 and 22) is an ocular emergency that requires prompt treatment and referral. A melting ulcer occurs when an infection from collagenase and matrix metalloproteinase (MMP) producing bacteria (or, rarely in the UK, fungi) such as Pseudomonas or Staphylococcus and Streptococcus species¹¹ causes rapid corneal stromal destruction. This is often worsened when leukocytes are released into the tear film, which also produce collagenases and MMPs, and speed up the destruction of stroma.
Melting ulcers usually have a diagnostic appearance, often presenting with an oedematous cornea that is gelatinous in appearance. These ulcers may be accompanied by a yellow to grey discharge and signs of ocular pain and reflex uveitis. Often, brachycephalic patients can appear less painful, and this could be attributed to their decreased corneal sensation.
Keratomalacia can develop rapidly and can risk perforating the eye in as little as 24 hours; therefore, prompt treatment or referral is needed to reduce the risk of further complications, or loss of the eye.
As these ulcers are a result of the enzymes that destroy stroma, all diagnostic tests should be performed for ulcers to rule out underlying causes that also need to be addressed, making sure to take care in cases where the ulcer is deep or the tissue is fragile. In these cases, samples for culture and sensitivity should be taken for tailored antibiotic therapy.
Medical treatment is often intense over the initial 24 to 48 hours and these patients often need to be hospitalised.
Melting ulcers are associated with significant stromal loss and may require surgical treatment (keratectomy of the remaining keratomalacia followed by placement of conjunctival flap, amnion graft, a cell graft or biosis graft). Enucleation is reserved for cases involving blind, painful eyes, extensive corneal defects/perforations, and situations where the owner cannot afford corneal surgery.
Corneal cross-linking (CXL) can be used for treatment of keratomalacia. It is a technique requiring specialist equipment. CXL is usually performed in the sedated or anaesthetised animal; however, if for any reason this is contraindicated, it can be performed consciously in an amenable patient. CXL increases biomechanical stability and strengthens the cornea. It can also sterilise the site of ulceration, killing off the bacteria that may be contributing to the melting process.
The corneal surface is primed with riboflavin, which is applied at a rate of one drop every 2 minutes for 30 minutes. The cornea is then flushed with sterile saline or balanced salt solution, and anaesthetised topically. The cornea is then irradiated using UV-A light.
The riboflavin absorbs the light and produces free radicals, which cause new bonds (cross-links) to form between adjacent collagen strands in the stromal layer12.
Recent studies revealed that argon cold atmospheric plasma (ACAP) and N-acetyl cysteine (NAC) demonstrate an in vitro antimicrobial activity. The results warrant a clinical pilot study to assess the potential of NAC and ACAP to reduce or replace the use of topical antibiotics13,14.
● A thorough work-up should be done on all ulcers to determine if an underlying cause exists.
● Referral should be considered for all complicated ulcers, as they require more intense observation and care.
● Deep ulcers, descemetoceles, corneal perforations and keratomalacia are ocular emergencies.
● These conditions are painful, so analgesia should be considered in all cases, and may require a multimodal approach to ensure patient comfort.
| Table 2. Further reading: recent articles related to corneal ulcers | |||
|---|---|---|---|
| Authors | Title | Summary/Conclusion | In vitro/in vivo |
| D Costa et al15 | “A multicenter retrospective study on cryopreserved amniotic membrane transplantation for the treatment of complicated corneal ulcers in the dog” | Cryopreserved amniotic membrane transplantation is an effective surgical technique for the treatment of complicated corneal ulcers in the dog, with highly satisfactory visual and cosmetic outcomes (99 per cent of cases) | In vivo |
| Cebrian et al16 | “Corneo-limbo-conjunctival transposition (CLCT) to treat deep and perforating corneal ulcers in dogs: a review of 418 eyes and corneal clarity scoring in 111 eyes” | CLCT in dogs is a good technique to treat deep ulcers and has a high corneal clarity score, but has a less desirable outcome when treating pre-existing perforations and pugs | In vivo |
| Keenan et al17 | “Corneoconjunctival transposition with and without ACell for deep corneal ulcer repair in 18 dogs” | Corneoconjunctival transposition with ACell can be used for corneal ulcer repair in dogs | In vivo |
| Dees et al18 | “Use of autologous serum or Vizoovet to improve healing rates of spontaneous chronic corneal epithelial defects after diamond burr debridement (DBD) in dogs” | The use of Vizoovet as adjunctive medical treatment resulted in a marginally shorter corneal healing time after DBD compared to ofloxacin alone, and ofloxacin and autologous serum. Comparing the adjunctive Vizoovet and autologous serum, the mean days until healed were 16.0 ± 3.7 days, and 16.3 ± 4.5 days, respectively. | In vivo |
| Yates et al19 | “In vitro antibacterial efficacy of autologous conditioned plasma (ACP) and amniotic membrane eye drops” | Canine and equine ACP partially inhibited Enterococcus faecalis growth in vitro. Further studies using varying concentrations of ACP against bacterial isolates from corneal ulcers are warranted. | In vitro |
| Tsvetanova et al20 | “Melting corneal ulcers (keratomalacia) in dogs: a 5-year clinical and microbiological study (2014-2018)” | The prevalent bacterial species linked to canine keratomalacia include Pseudomonas aeruginosa and β-haemolytic Streptococcus species. It is advisable to conduct bacterial culture and sensitivity testing for all dogs exhibiting keratomalacia. Notably, corneal ulcers that result from a pure Pseudomonas species infection have a significantly higher likelihood of leading to globe loss compared to ulcers associated with other bacterial cultures. | In vivo |