17 Sept 2018
Gerald McLaughlan, in the first of a two-part article, looks at some of the most common procedures performed in canine and feline patients.
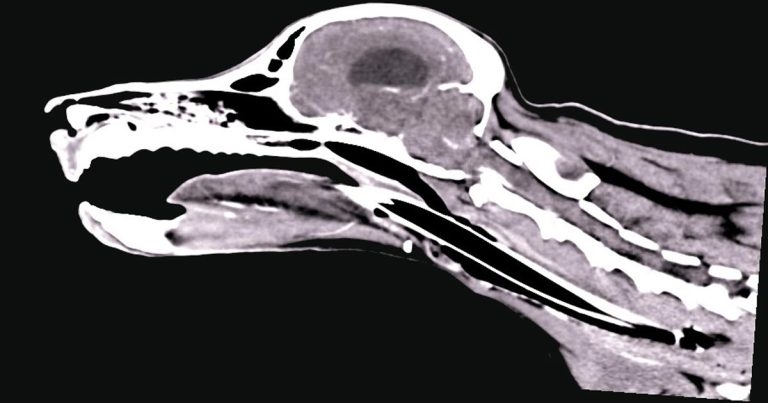
Figure 2. A sagittal CT image showing a typical appearance of a nasopharyngeal stenosis (note the small band of tissue crossing the nasopharynx).
Interventional radiology (IR) involves use of contemporaneous imaging modalities, such as fluoroscopy, bronchoscopy and cystoscopy, to undertake minimally invasive procedures.
Using a combination of percutaneous access and natural orifices, the clinician can target the required organ and deploy devices to relieve obstruction or administer targeted drug therapy. This article will highlight some of the most common IR procedures the author performs.
Tracheal collapse (Figure 1) is a progressive, debilitating disease that primarily affects middle-aged, small breed dogs, with Yorkshire terriers and Pomeranians being over-represented in particular. The disease is due to degeneration of the tracheal cartilage, combined with reduced glycosaminoglycan/loss of elastic fibres of the tracheal membrane, which results in dorsoventral flattening.
While tracheal collapse is initially focal, due to its progressive nature it may become diffuse, and can ultimately involve the entire trachea and lower airways. Clinically, the disease is characterised by a classic “goose honking” cough (often exercise or excitement-induced) and periods of cyanosis.
While initially medical management with a combination of antitussives, anti-inflammatory steroids, bronchodilators and anxiolytics can be successful, many dogs will require interventional procedures as the disease progresses (approximately 70% of cases will remain asymptomatic with medical management for more than 12 months).
Tracheal stenting is largely considered a salvage procedure and should only be performed when medical management has been exhausted. Clients should be aware that, following stent placement, concurrent life-long medical management will likely be required. Tracheal stenting is ideally performed using a combination of bronchoscopy and fluoroscopy.
While reports exist of the procedure being performed using digital radiography, this is not recommended. Under anaesthesia, the author performs full tracheobronchoscopy and obtains a bronchoalveolar wash to submit for cytology and culture. Following this, the patient is placed in lateral recumbency and a radiographic marker catheter is placed down the oesophagus to allow measurement of maximal tracheal diameters under positive pressure ventilation and tracheal length (the marker catheter is used to account for any magnification of the fluoroscopy unit).
Tracheal stents are composed of nitinol (nickel-titanium) and a range of self-expanding or balloon-expandable stents are commercially available. While tracheal collapse can be focal (most commonly seen at the thoracic inlet), due to its progressive nature it is recommended the entire length of the trachea, from 1cm caudal to the larynx to 1cm cranial to the carina, is stented. The effectiveness of bronchial stenting in patients with concurrent bronchial collapse is the subject of debate.
Tracheal stenting should only be performed by those trained in the technique, as many of the reported complications are due to inappropriate measurements and stent sizing. Complications include stent migration, stent fracture, collapse above or below the stent, infection and granulation tissue formation. Work has suggested around 40% of cases may require placement of a second stent at some point in their lifetime (Weisse and Berent, 2015).
Overall, clinical improvement and owner satisfaction rates post-tracheal stenting is considered excellent.
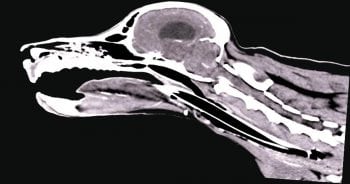
Nasopharyngeal stenosis (NPS; Figure 2) is a pathological narrowing within the nasopharynx caudal to the choanal. Clinical signs associated with this include both inspiratory and expiratory stertor, along with recurrent nasal discharge/infection.
While NPS can be a congenital abnormality, the majority of cases occur following aspiration rhinitis as a complication of general anaesthesia. Surgical options for NPS are associated with relatively high recurrence rates, so minimally invasive fluoroscopic assisted procedures are regarded as the gold standard.
Options for NPS include balloon dilation or placement of a stent. Balloon dilation and stent placement should only be considered after a contrast CT scan of the area is performed to allow accurate measuring and precise identification of the stenosis.
The stenosis is most commonly located at the junction of the hard and soft palate,but can occur both rostral and caudal to this.
For very thin lesions (less than 0.5cm), multiple balloon dilations alone may be successful. However, for lesions more than 0.5cm, recurrent lesions or complete stenosis, balloon dilation alone is unlikely to be successful and placement of a nasopharyngeal stent should be recommended.
Placement of a stent for NPS is performed with the patient in lateral recumbency using a combination of endoscopy and fluoroscopy.
This allows for accurate identification of the stricture and ensures the stent is located in the correct position (across the stenosis, but avoiding placement into one of the nasal passages as this results in excessive mucous accumulation due to compromised drainage and too caudal in the nasopharynx as this can result in irritation and reflux).
Options for NPS stents include balloon expandable and self-expanding. The stents can either be covered (stronger, but risk of migration and chronic infection) or uncovered. Complications associated with NPS stenting are rare, but include restenosis, stent compression, stent migration, chronic infection and development of an oronasal fistula. The prognosis for dogs and cats with NPS is considered good to excellent.
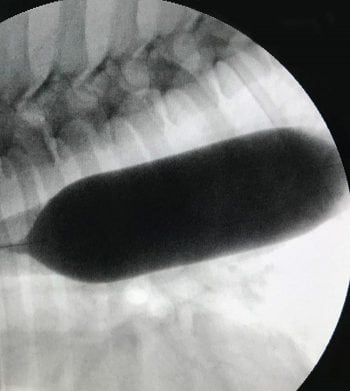
Oesophageal strictures (Figures 3 and 4) occur in both dogs and cats, and are associated with clinical signs including regurgitation, hypersalivation and dysphagia. In dogs, they most commonly occur following recent anaesthesia, which results in gastro-oesophageal reflux and stricture formation. In cats, particular care should be taken when administering doxycycline as this has been associated with development of oesophageal strictures. Options for oesophageal strictures include balloon dilation, bougienage and oesophageal stenting.
The author’s preferred treatment for oesophageal strictures is balloon dilation using a combination of fluoroscopic and endoscopic visualisation. With many oesophageal strictures, a muscular component to the fibrosis exists, and with endoscopy, you are only monitoring the mucosal/submucosal tearing, whereas with fluoroscopy, you are able to monitor and ensure the entire stricture “belt” is torn.
Maximal oesophageal diameter and stricture length is also best measured using contrast fluoroscopy. It is generally recommended to start with a balloon 2mm to 4mm greater than the stricture diameter and then gradually increase the balloon size by 2mm to 4mm until maximal oesophageal diameter is achieved and the stricture “belt” is completely effaced.
Once the stricture is engaged, the author prefers to leave each balloon inflated for 3min to 5min. Prior to balloon dilation, some clinicians may consider injection of triamcinolone into the stricture, which may limit stricture recurrence.
The number of balloon dilations required is, on average, two to four, although significantly more may be needed in severely refractory strictures. No standard recommendation exists regarding the interval that should be recommended between balloon dilations,but, in general, seven days would be considered appropriate.
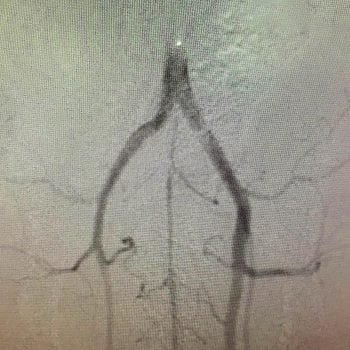
In cases of refractory oesophageal strictures, consideration should be given to placement of an indwelling balloon oesophageal (BE) tube. The tube is placed under fluoroscopic guidance, remains in place for six weeks and allows the owners to inflate the balloon twice daily at home to prevent stricture reformation.
The BE tube also allows tube feeding, although oral feeding is to be encouraged. The indwelling BE tube has, in the author’s opinion, made use of the oesophageal stent redundant in most cases of benign oesophageal stricture.
Increasing interest has occurred in targeted intra-arterial (IA) chemotherapy (Figure 5) in veterinary patients in recent years.
In particular, most research and clinical work has been focused on non-resectable hepatocellular carcinomas and urinary tract neoplasia (urothelial cell carcinomas and prostatic carcinoma). This involved the patient being anaesthetised and vascular access being obtained via the femoral or carotid artery.
Using fluoroscopic guidance, the clinicians selectively deliver the chemotherapeutic agent to the tumour via its arterial supply. The IA administration of chemotherapy agents is not associated with an increased level of drug-associated side effects.
Regarding urinary tract tumours, various studies have shown higher local chemotherapy concentrations and improved remission rates in laboratory animals (a study in research beagles documented it was possible to achieve an eight-fold increase in bladder concentration of the chemotherapy agent compared to dogs receiving the drug via the traditional IV route).
IA chemotherapy has been shown to result in increased tumour drug concentration and may, therefore, result in increased efficacy.
The author has commonly been treating bladder, urethral and prostatic neoplasia with intra-arterial chemotherapy alongside other modalities, including surgery, IV chemotherapy and radiotherapy, with a positive response seen.
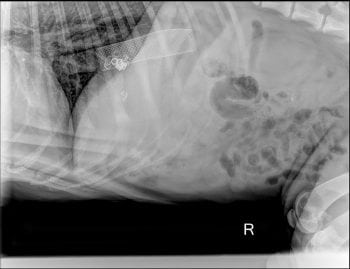
Solitary hepatocellular carcinomas (HCCs) should be regarded as a surgical disease in dogs (median survival of more than three years following complete excision).
However, in patients where the tumour is considered non-resectable, nodular or diffuse – or in which owners decline surgery – transarterial chemoembolisation is an option.
This technique has been performed for several years and offers a reasonable outcome in cases where surgery is not appropriateor declined.
As HCCs receive almost their entire blood supply from the hepatic artery, whereas the normal liver receives most of its supply from the portal vein, it makes them particularly sensitive to arterial chemoembolisation.
Access is gained via the femoral artery under fluoroscopic guidance, followed by selection of the coeliac and common hepatic artery. After this, super selection of the arterial branches supplying the tumour is achieved to ensure minimal non-target embolisation occurs.
The chemotherapy and embolisation agent (lipiodol or polyvinyl alcohol particles) are administered in this location and a repeat contrast fluoroscopy study is performed to confirm reduction in blood flow to the tumour.
Following chemoembolisation of a non-resectable HCC, the tumour does generally not shrink significantly (less than 30%) and the general aim is “stable disease”. The procedure may need to be repeated multiple times to limit further tumour growth.
Surgical correction of portosystemic shunts is widely recognised as preferable to medical management regarding long-term outcome.
However, perioperative complication and mortality rates associated with attenuation of an intrahepatic portosystemic shunt has resulted in development of a minimally invasive option for these vascular abnormalities, called percutaneous transvenous embolisation (PTE).
Prior to PTE, the patient should be medically managed with a combination of diet, antibiotics and lactulose for at least two to four weeks. The author also starts levetiracetam (20mg/kg three times a day, used under the cascade) 72 hours prior to any intrahepatic or extrahepatic portosystemic shunt procedure.
PTE involves using jugular access to deploy a stent within the vena cava across the shunt entrance (maximal caval diameter and shunt length determined either by pre-procedure CT scan on intra-procedure fluoroscopy). Care must be taken to measure the caval pressure (central venous pressure) and portal pressures at all times to ensure portal hypertension does not develop.
Following stent placement, a hydrophilic guide wire and catheter combination is used to access the shunting vessel through the stent and deploy stainless steel coils (typically around 12 per case, with a range of 1 to 18 in a recent study). During coil deployment, the clinician must continually monitor portal pressures to ensure they don’t increase by more than 7mmHg from the initial value (or more than 16mmHg as a maximum value).
The coils are thrombogenic and will result in gradual further occlusion of the shunt over the following weeks, and allow gradual withdrawal of medication and dietary management.
PTE is a safe, fast and effective technique when performed by a clinician with appropriate training. It offers a similar long-term outcome to traditional surgical options, but with reduced morbidity and mortality (Figure 6).
Ectopic ureters (Figure 7) are a congenital abnormality most commonly found in female dogs. Certain breeds are over-represented,too, including the golden retriever andLabrador retriever.
As this is a congenital abnormality, these cases typically present around three to five months of age due to a perceived lack of house training. The incontinence is typically continuous in nature and concurrent urinary tract infection is common.
The ability of the patient to also pass a normal stream of urine may indicate the ectopic is unilateral rather than bilateral. Male ectopic ureters are less commonly associated with incontinence as there is typically a stenosis at the distal ureter meaning that these cases are normally found to have massive hydroureter and hydronephrosis on ultrasound.
Traditional surgical re-implantation is associated with various complications (25% to 50% developing worsening hydroureter/hydronephrosis and 14% developing uroabdomen). Cystoscopic laser-guided ablation of ectopic ureters provides a minimally invasive alternative in both males and females.
The procedure is performed on an outpatient basis and associated with similar improvement in continence score when compared to reimplantation. At the same time, the author also performs laser correction of any vestibulovaginal remnants as they may contribute to ongoing incontinence or recurrent infections.
Ureteral and urethral obstructions can be caused by both benign and malignant disease. Ureteral obstruction is most commonly seen in cats due to calcium oxalate ureterolithiasis and urethral obstructions are most commonly seen in dogs due to urogenital neoplasia.
Ureteral obstructions in cats are best addressed with placement of an SC ureteral bypass (SUB) device under fluoroscopic guidance (Figure 8). The SUB device is associated with significantly lower mortality than traditional ureteral surgery.
The major complication associated with SUB placement is obstruction of the device, which occurs in around 25% of cases (however, only 12.5% of cases require device exchange as the previous ureteral obstruction has resolved; Berent and Weisse, 2018).
In cats with ureteral obstruction secondary to calcium oxalate stones, owner education on stone management/preventive factors is essential.
Ureteral stents in cats are poorly tolerated in particular with regards to post-placement dysuria, which is reported in up to 35% of cases (Berent et al, 2014). In dogs, ureteral obstructions are best managed with a cytoscopically placed ureteral stent (Figure 9; Pavia et al, 2018).
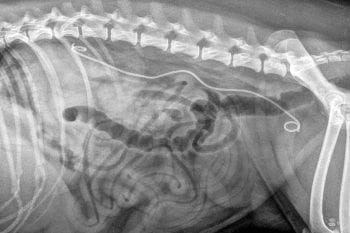
Urethral obstructions occur much more commonly in dogs than cats and are largely due to neoplastic disease of the bladder, urethra or prostate. If bladder tumours are apical, they are best managed with surgical resection; however, the majority are trigonaland, therefore, can resultin obstruction.
Urethral stents are placed using fluoroscopy on an outpatient basis when the patient is showing clinical signs of obstruction (rather than dysuria, but still passing good streams of urine).
Urethral stents can be placed quickly and result in immediate relief of the obstruction.
The major complication seen following urethral stent placement is incontinence (25% of cases) – the location and length/diameter of the stent were not associated with the development of incontinence in one study, although, if possible, the author avoids crossing the trigone withthe stent.
In cases with malignant obstructions, the mean survival time following stent placement in patients receiving chemotherapy was 270 days (Blackburn et al, 2013).