27 May 2019
Jo Murrell discusses types, uses and latest developments in canine and feline patients, including endotracheal tubes.
The recent shortage of isoflurane has focused the attention of veterinary practitioners on other techniques to maintain anaesthesia in cats and dogs.
Use of inhaled sevoflurane and total IV anaesthesia (TIVA) techniques are the alternatives to inhaled isoflurane in modern veterinary practice. Combinations of medetomidine or dexmedetomidine and ketamine (plus an opioid) are also widely used for shorter procedures such as neutering, but will not be discussed in this article.
This article will compare inhalant and TIVA techniques, as well as comparing isoflurane and sevoflurane for maintenance of anaesthesia. It will also cover some new developments in companion animal anaesthesia, such as the recent release of multidose alfaxalone. Different types of endotracheal (ET) tubes, and their advantages and limitations, will also be discussed.
Both propofol and alfaxalone are suitable for maintenance of anaesthesia using TIVA, and a large number of publications exist that describe the use of these drugs for maintenance of anaesthesia in dogs and cats (for example, Ilkiw and Pascoe, 2003; Schwarz et al, 2014; Dehuisser et al, 2017; Bustamante et al, 2018).
It is noteworthy that alfaxalone is licensed for maintenance of anaesthesia for up to 60 minutes’ duration. The formulation of propofol that contains preservative should not be used for TIVA because of the risk of benzyl alcohol toxicity. It is important to remember neither propofol or alfaxalone has analgesic properties, therefore premedication with an opioid drug is vital to ensure adequate analgesia during the procedure and reduce the infusion rate of the hypnotic agent, thereby contributing to a balanced anaesthesia technique.
Using a full µ agonist for premedication, such as methadone, should have a greater drug sparing effect than premedication with buprenorphine. Similarly, premedication with a potent hypnotic drug, such as an alpha 2 adrenergic agonist (for example, medetomidine), will have a greater drug sparing effect on the TIVA infusion than premedication with acepromazine (Herbert et al, 2013), which may be advantageous in terms of stability of anaesthesia using the TIVA technique.
Propofol and alfaxalone can be given by repeated bolus administration to maintain anaesthesia, but this leads to peaks and troughs in anaesthetic drug concentration that can be associated with a lightening of anaesthesia, or, conversely, respiratory and cardiovascular depression when the concentration of anaesthetic drugs are high. However, a more elegant way to deliver TIVA is via a constant rate infusion (CRI) using a controlled infusion apparatus, such as a syringe driver. Using a CRI will likely reduce the overall dose of propofol or alfaxalone administered and lead to a more stable plane of anaesthesia, with greater stability of physiological variables throughout surgery. In human anaesthesia, where the pharmacokinetics of propofol in a large population of patients have been established, there has been the development of a target controlled infusion system for propofol (Glen, 1998).
Programmed pharmacokinetic parameters for propofol are used to deliver propofol to a target plasma concentration in the individual patient, based on the patient’s characteristics, such as bodyweight. A similar system has been developed for dogs by the University of Glasgow (Musk and Flaherty, 2007; Auckburally et al, 2008), although it is not widely available in veterinary practice.
Propofol is a phenol, and due to the limited ability of cats to conjugate with glucuronide (van Beusekom et al, 2015), accumulation of propofol and prolonged recoveries are seen in cats given longer duration infusions with propofol (Pascoe et al, 2006), which are, therefore, not recommended.

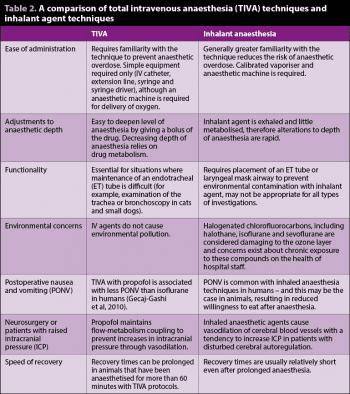
Doses for maintenance of anaesthesia by CRI for propofol and alfaxalone in premedicated cats and dogs are given in Table 1, although it is important to remember, similar to inhaled anaesthesia techniques, each animal is an individual and doses may vary depending on the individual, type of surgery and premedication drugs used. The maintenance dose is started after an induction dose of the chosen hypnotic agent has been given. Similarly to inhaled anaesthesia techniques, maintenance of a secure airway using an ET tube or laryngeal mask airway (LMA) should be considered mandatory for TIVA techniques. Oxygen supplementation (more than 30% FiO2) should also be provided to prevent hypoxaemia should respiratory depression occur during anaesthesia. Differences between TIVA and inhaled anaesthesia techniques are given in Table 2.
Alfaxalone multidose has been released as an alternative to a preservative-free preparation, which is likely to become unavailable with time. The new preparation has a 28-day shelf life once the bottle is broached, which overcomes the limitation of having to discard the bottle after single use. The preservatives in the multidose preparation are ethanol, chlorocresol and benzethonium chloride, therefore, there are not the concerns about benzyl alcohol toxicity seen with propofol when using the multidose preparation for TIVA protocols. The pharmacokinetics of the single use preparation and multidose preparation have been shown to be similar in dogs and cats.
Many vets with access to sevoflurane vaporisers turned to sevoflurane during the recent shortage of isoflurane, but what are the differences between these two inhalant agents?
Both agents are halogenated chlorofluorocarbons that can only be used in agent-specific dedicated vaporisers, to ensure accurate delivery of inhalant agent to the patient. Differences and similarities between the two inhalant agents are outlined in Table 3.
Securing the airway to ensure a robust supply of oxygen and exhalation of carbon dioxide to the anaesthetised patient is fundamental to good patient care during anaesthesia. It is also important to prevent aspiration of fluid should regurgitation occur or prevent aspiration during procedures where fluids such as blood or debris (such as during a dental) may be inhaled. However, ET intubation is not without complications – particularly in cats – and ET intubation was associated with a two-fold increase in odds of death in cats in the Confidential Enquiry into Small Animal Peri-operative Fatalities study (Brodbelt et al, 2007). Such complications include tracheal rupture, tracheal stricture, trauma to the arytenoids and laryngospasm.
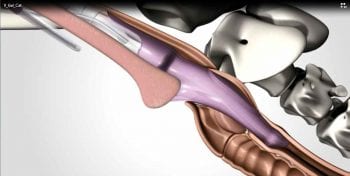
Supraglottic airway devices, such as the laryngeal mask airway, were first developed for use in humans to avoid complications associated with ET intubation, and have been shown to be easy to place and an effective means to secure an airway. However, LMAs may not effectively protect against aspiration should regurgitation occur. To combat complications associated with ET intubation in cats a supraglottic airway device has been developed for this species termed the v-gel (Figure 1).
The v-gel has a sophisticated design to ensure a good airway seal around the pharyngeal, laryngeal, perilaryngeal and upper oesophageal structures. An inflatable dorsal pressure adjuster is found on the dorsal surface of the v-gel that is attached to a valve to allow inflation of this region of the device to ensure a good seal around the pharynx (Figure 2).
A large airway channel is seen on the ventral surface to minimise the increase in airway resistance that can occur with small diameter ET tubes. The airway channel is surrounded by a cuff also, to ensure a good seal with the trachea to reduce the risk of aspiration of regurgitant or foreign material. There is also an integrated port for connection of a gas sampling line to allow monitoring using capnography, without the addition of another connector that would otherwise add dead space.
A number of studies exist that have evaluated the v-gel in cats (van Oostrom et al, 2013; Barletta et al, 2015; Prasse et al, 2016). One study has shown intubation with a v-gel requires a lower dose of propofol than ET intubation (Barletta et al, 2015), although this finding was not repeated in another study (van Oostrom et al, 2013).
Differences between the two results probably stem from the fact students were intubating the cats in the study where the difference in propofol dose was found. Furthermore, inexperienced veterinary students took less time and fewer attempts to successfully place the v-gel compared to an ET tube (Barletta et al, 2015). In this study (Barletta et al, 2015), laryngeal sensitivity was reduced and willingness to eat after anaesthesia was also enhanced in the v-gel group. Concerns have been raised about the v-gel and the efficacy of the seal around the larynx to prevent leakage of gases during intermittent positive-pressure ventilation (IPPV) or to prevent aspiration should regurgitation occur.
Leakage may occur if the v-gel becomes malpositioned during movement of the cat – although should resolve with repositioning of the device (Barletta et al, 2015). However, another study showed during the v-gel and LMA was associated with less leakage around the device than an ET tube with an inflated cuff (to 20cmH20) during IPPV (Prasse et al, 2016). Dislodgement of the v-gel during movement of the patient resulting in a poor seal or airway obstruction is a concern, and correct placement should always be confirmed using capnometry continuously during anaesthesia when a v-gel is the chosen means of maintaining the airway.
I
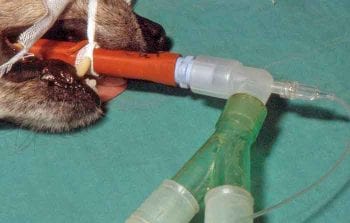
nflation of the cuff on an ET tube is recommended to prevent aspiration of material, reduce environmental contamination with inhalant agents and more easily allow IPPV if necessary. Two types of ET tube cuffs are available – low-pressure, high-volume cuffs that have a large area of contact of the cuff with the trachea, and high pressure low volume cuffs that exert a larger force against the wall of the trachea are probably better at preventing aspiration, but also pose a much greater risk of tracheal necrosis.
Older style red rubber tubes have a high-pressure, low-volume cuff, and use in cats and dogs should be discontinued (Figure 3). Modern PVC and silicone tubes have low-pressure, high-volume cuffs and their use is recommended in companion animals.
Different brands of tubes may have slightly different shaped cuffs that may affect contact with tracheal mucosa and the risk of necrosis or aspiration should regurgitation occur. However, no studies exist evaluating the comparative safety and efficacy of different cuff designs in veterinary patients.
Different techniques have been proposed for cuff inflation to reduce the risk of tracheal necrosis following intubation. The simplest technique is for one person to listen for a leak around the tube, while a second person gently squeezes the reservoir bag on the anaesthetic breathing system. The cuff is gently inflated until no further leak can be heard. An alternative technique is to inflate the cuff until a predetermined degree of inflation of the pilot balloon is reached. However, both of these techniques have been shown to produce high pressures in the cuff of the ET tube that may be associated with the potential for tracheal damage (Bird et al, 2019).
In human anaesthesia, it is recommended to inflate the cuff using a pressure gauge to measure intra-cuff pressure and to inflate the cuff to a pressure between 20cmH2O to 30cmH2O (Morris et al, 2007). Although this is likely to be a safer technique than the aforementioned methods, it is noteworthy the safe cuff pressures for veterinary patients, particularly cats, have not been determined.

Armoured ET tubes have a spiral of wire running through their wall, which makes them virtually impossible to kink (Figure 4). They should be selected when the positioning of the patient makes kinking of the ET tube and, therefore, loss of a patent airway a possibility – for example, during surgery for brachycephalic obstructive airway syndrome or some complex dental procedures. The thickness of the wall of the tube means a slightly smaller internal diameter ET tube needs to be selected for a given patient, compared to a standard ET tube.
The shortage of isoflurane brought the reliance of the veterinary profession on isoflurane for maintenance of anaesthesia into the spotlight. However, different techniques for maintenance of anaesthesia – including TIVA and use of inhaled sevoflurane – are practical alternatives to isoflurane and should be considered, particularly for short duration procedures.
Supraglottic airway devices are now available for cats and may offer certain advantages over ET intubation; however they should always be used with capnometry to confirm correct placement of the device in the airway without the risk of airway obstruction or a poor seal leading to aspiration.