12 Oct 2021
Emi Barker BSc(Hons), BVSc(Hons), PhD, DipECVIM-CA, MRCVS, discusses treatment, diagnosis and containment options for this acute, contagious cause of cough in dogs.

Canine infectious respiratory disease complex is an acute, contagious cause of cough caused by a variety of bacteria and viruses. It is commonly known as kennel cough, although this does not reflect its frequent finding in dogs that have not been kennelled nor the variety of clinical signs that may accompany infection by its various contributory pathogens. In most cases, management is focused on treatment of the clinical signs and minimisation of spread to others. It is possible to vaccinate against many of the agents involved.
Canine infectious respiratory disease complex (CIRDC) is a common cause of acute-onset cough in dogs. It is commonly known as kennel cough or infectious tracheobronchitis; however, many dogs present without a history of kennelling, while clinical signs frequently extend beyond the larger airways.
Understanding the properties of the underlying pathogens is helpful in considering the management required to control infection.
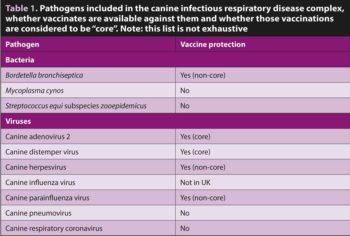
Causative agents include bacteria and viruses (Table 1). Infections are considered to be synergistic in their pathogenicity, with some only associated with clinical signs when present with other agents1,2.
Following a period of incubation (typically lasting from a few days up to around 10), clinical signs may be seen and the pathogen shed into the environment. For some CIRDC pathogens (for example, canine distemper virus; Bordetella bronchiseptica) shedding can continue for a few months following resolution of clinical signs, whereas shedding of most other viral agents will cease within a couple of weeks. This has implications for the ongoing management of these cases, in minimising the risk of spread to others.
For most CIRDC pathogens direct airborne transmission is considered the most common route of infection between dogs. Most viruses survive poorly outside of the host; however, canine adenovirus 2 (CAV-2), by virtue of being a non-enveloped DNA virus, can survive for weeks to months under ideal conditions. Similarly, B bronchiseptica, and likely Mycoplasma cynos, can survive for weeks to a few months under their ideal conditions (for example, moist environment). Such environmental persistence can result in indirect transmission to naive individuals and propagation within kennelling situations.
Of the bacteria, B bronchiseptica is most frequently detected, being found in approximately 75% to 80% of dogs with infectious cough1. Following inhalation, Bordetella adhere to the respiratory cilia, where they can evade the majority of the host immune response and replicate. They produce tracheal cytotoxin and dermonecrotic toxin leading to tracheal damage and inflammation.
Infection with B bronchiseptica can also cause disease in other species, including rodents, cats and humans. M cynos and other Mycoplasma species (for example, Mycoplasma canis) have been detected at various levels within the canine respiratory tract of clinically normal, as well as in coughing, dogs. There is most support for M cynos being pathogenic in the lower airways, with less support for other Mycoplasma species having a significant role in clinical signs3.
Another bacterium, Streptococcus equi subspecies zooepidemicus, has more recently been recognised as a component of CIRDC4. Although it can be detected in clinically healthy dogs, it is more frequently encountered in those of increasing disease severity. In some cases, infection can progress to haemorrhagic streptococcal pneumonia.
Other bacteria that have been detected in the airways of dogs with respiratory disease (for example, Pasteurella species) are suspected to be opportunistic secondary invaders.
Of the viruses, canine parainfluenza virus (CiPV) is most prevalent, being detected in approximately one-third of dogs with infectious cough1. In isolation, infection with CPiV results in very mild, if apparent, clinical signs. Similarly, CAV-2, which primarily infects the upper respiratory tract, is associated with mild respiratory signs in isolation.
CAV-2 has rarely been suspected of causing gastrointestinal or neurological disease. CAV-1, the agent of canine infectious hepatitis, may also induce respiratory signs. Canine influenza virus (CIV) emerged in the early ’00s as a cause of severe respiratory disease (haemorrhagic pneumonia with a high mortality) in dogs housed together (that is, racing greyhounds; fox hounds). Most outbreaks have been linked to contact with horses, reflecting the close relationship between CIV and equine influenza virus.
The role of canine herpesvirus (CHV-1) in CIRDC is unclear2. It is unlikely to play a significant primary role in CIRDC; however, recrudescence of infection exacerbates concurrent disease caused by other pathogens within the complex. Canine respiratory coronavirus (CRCoV) is another virus that emerged in the early ‘00s, where it can predispose dogs to secondary infections, and ultimately clinical disease, by damaging the respiratory mucosa.
Many individual dogs presenting with CIRDC will have a recent known history of exposure to another dog with cough or, at least, mixing with unfamiliar dogs (for example, recent kennelling). While any situation in which a transient population of dogs is present has an increased risk of an outbreak of CIRDC, this includes boarding kennels, rescue shelters, dog shows and veterinary hospitals.
One study reported that around 90% of dogs developed signs of CIRDC within three weeks of entering a rehoming shelter5. It is also telling that the newer additions to the CIRDC complex (for example, CIV or CRCoV) were first detected during outbreaks of respiratory disease within kennelling situations. The continuous introduction and mixing of naive dogs that are likely to be experiencing considerable stress into a high-stocking density environment containing dogs potentially shedding multiple respiratory pathogens propagates the problem.
Any concurrent respiratory disease (including chronic bronchitis, bronchiectasis and tracheal collapse) that impairs mucociliary clearance of the lower airways will increase the risk of CIRDC. Similarly, in dogs with history or diagnostic evidence of chronic respiratory disease alongside an acute presentation or deterioration in clinical signs, a concurrent infectious process should be considered, as infections could represent a treatable process.
As vaccines are available for many of the more significant CIRDC pathogens, an incomplete vaccine history is a significant risk factor. For some CIRDC pathogens young age is also a risk factor for infection, likely reflecting a combination of limited immunity (which is subsequently acquired through vaccination or environmental exposure) and increased likelihood of kennelling or mixing with others.
Dogs typically present with an acute-onset paroxysmal cough, which is often non-productive in the earlier stages – that is, “a dry hacking cough”. This may progress to a productive cough, although many owners struggle to differentiate a truly productive cough from one associated with the regurgitation of saliva. Some dogs may also cough to the point of vomiting.
Other clinical signs include ocular and nasal discharge, inappetence to anorexia, lethargy, pyrexia, and potential progression to pneumonia and death. Some causative pathogens may also have effects outside of the respiratory tract (for example, gastrointestinal and/or neurological signs in dogs with distemper; keratitis in dogs with CHV-1).
Most dogs can be presumptively diagnosed with CIRDC based on their clinical history and physical examination findings. Further investigation is rarely indicated unless the clinical signs are prolonged, clinical signs are severe (for example, bacterial bronchopneumonia is suspected), if another disease process is considered to be likely (for example, tracheal foreign body following acute-onset cough in a dog immediately following running through long grass), or if multiple dogs in a kennelling or hospital situation are affected6.
For individual dogs, a thorough physical examination is important; primarily to assess for the severity of disease that would indicate the need to intervene sooner rather than later (for example, alterations in the thoracic resonance that could suggest consolidation or pneumothorax, crackles that could suggest the presence of fluid in the airways, marked pyrexia) and to look for evidence of concurrent clinical disease that could impact on the disease process, investigation or treatment (that is, heart murmur).
“Routine” blood analysis (haematology and serum biochemistry) is poorly sensitive for most forms of respiratory disease, but it can be useful in indicating the presence of a severe disease process (for example, neutrophilia or neutropenia, left-shift or toxic change) and increase the suspicion of other differentials (for example, eosinophilia in cases of parasitism or eosinophilic bronchopneumopathy).
Routine blood analysis is usually more helpful in excluding co-morbidities that could impact on investigation or treatment, as well as providing base-line results prior to treatment. Dependent on prophylaxis administered, Angiostrongylus serology should be considered.
Thoracic imaging is also of benefit, to assess those dogs that are particularly severely affected and those for whom other differentials are possible.
CT imaging is preferable, particularly if a radiolucent foreign body is a differential; however, where this modality is not available, three-view radiographs would be an alternative option. In cases of CIRDC, minimal to no change may be seen on CT or radiographs, through to evidence of fulminant pneumonia may also be present in severely affected cases.
Anaesthesia is often preferable to heavy sedation, as these dogs are already respiratory compromised, may be at increased risk of aspiration (for example, if they have a history of regurgitation) and induction anaesthesia permits evaluation of laryngeal function, as well as subsequent bronchoscopy and/or bronchoalveolar lavage (BAL).
Even where bronchoscopy is not available, a BAL (Panel 1) can be very useful. The BAL should be submitted for cytology, culture and respiratory pathogen PCRs (for B bronchiseptica and M cynos as a minimum). In CIRDC, BAL cytology typically reveals neutrophilic inflammation, with or without the presence of bacteria. B bronchiseptica organisms can sometimes be seen in BAL fluid samples as a Gram-negative coccobacilli (that is, short rods) adhered to ciliated epithelial cells7; however, this is not a specific finding.
While culture is possible in some dogs infected with Bordetella and this process can be useful in revealing antimicrobial resistance patterns, around 40% of dogs with confirmed infection are culture negative7. Culture of mycoplasmas under routine conditions is even less successful, primarily due to their slower growth rate and preference for enriched media. Therefore, PCR remains the modality of choice for the detection of both Bordetella and Mycoplasma.
In the face of an outbreak, where alternative non-infectious differentials are less likely, samples should be submitted for an extended respiratory pathogen panel; however, different panels are offered by different laboratories. Most panels include PCRs for canine parainfluenza virus and B bronchiseptica, while there is variable inclusion of PCRs for canine adenovirus, canine distemper virus, canine herpesvirus, canine influenza virus, S equi species zooepidemicus and M cynos.
Selection of the panel is in part dependent on the population affected; for example, if vaccination status is unknown or incomplete then inclusion of canine adenovirus and canine distemper virus would be important, while in vaccinated dogs that have close contact to equine stables canine influenza virus would be sensible.
Samples should be collected from multiple dogs and may comprise deep pharyngeal swabs (that is, that could be collected from a large number of dogs simultaneously), tracheal washes and BAL.
A complication in attribution of clinical signs to an individual pathogen detected is their presence in a significant number of clinically healthy dogs. In one study, approximately 45% of healthy dogs were positive for B bronchiseptica, 8% were positive for canine parainfluenza virus and one dog was positive for canine adenovirus 21.
Another study found no correlation between the antibody response to B bronchiseptica and the development of clinical signs, which is suggestive of a commensal state5. This is why samples from multiple dogs in an outbreak should be collected and an open mind maintained. Wherever possible, PCRs should be quantitative8.
For most dogs with uncomplicated CIRDC (that is, no known comorbidities; bright, normothermic and with a normal appetite) clinical signs will resolve within 10 days without antimicrobials6 – therefore, antimicrobials are not recommended. In these dogs, anti-tussives (for example, codeine), bronchodilators (for example, theophylline, terbutaline) and anti-inflammatory medication (for example, meloxicam) may be considered. Anti-tussives should not be used where bronchopneumonia Is suspected.
In dogs at risk of developing pneumonia (for example, those that are immunocompromised or have structural airway disease) or that are systemically unwell (for example, pyrexic, lethargic or inappetent) antimicrobials could reasonably be considered. Due to its activity against both B bronchiseptica and Mycoplasma species, doxycycline (5mg/kg orally twice daily) is the first-line option.
Where doxycycline is contraindicated, potentiated amoxicillin (12.5mg/kg orally twice daily) could also be considered, but is less desirable due to lack of activity against mycoplasmas, frequent resistance of B bronchiseptica to amoxicillin and increased selection for resistance in other organisms. Empirical treatment should not exceed 7 to 10 days.
Where a diagnosis of chronic B bronchiseptica infection has been made, and where culture results are unavailable, doxycycline is still the first-line option. Where mycoplasmas are suspected of contributing to clinical signs doxycycline is also the first-line option, with fluoroquinolones considered to be second-line options. If clinical signs improve within the first week, treatment should be continued for one week beyond resolution of clinical signs. If no improvement is noted a non-infectious cause of cough should be investigated.
In an outbreak setting, knowledge of the underlying pathogens could indicate the likely period of shedding following infection, the likelihood of environmental contamination and which antiseptic agents have activity against them. Exemplary hygiene measures need to be maintained to minimise risks of transmission to other dogs. If at all possible, no further dogs should be admitted to the facility.
Dogs with clinical signs, and those recently recovered, should be isolated, while anti-tussives may also reduce the likelihood of transmission. Deep pharyngeal PCRs could be considered to monitor for resolution of shedding prior to return to the general population.
It should also be borne in mind that cats too may become infected with B bronchiseptica if shared facilities are used. In the veterinary clinic: affected wards should be closed to incoming patients and, once empty, decontaminated; where possible single-use anaesthetic equipment should be used and anaesthetic breathing systems considered contaminated following use pending cleaning (using an agent with activity against the pathogen[s] known or suspected to be present). Dogs suspected of having an infectious cause of cough should be isolated from others.
The risk of clinical disease caused by many of the pathogens associated with CIRDC can be reduced using a combination of core and non-core vaccines9. Despite this, CIRDC remains common and vaccine uptake limited, especially against B bronchiseptica. Apart from for canine distemper virus, immunity is non-sterile, and it is possible for vaccinated dogs to become sub-clinically infected and transmit infection.
Although their intended use was not in the management of clinical respiratory signs, immunisation using parenterally administered modified-live-virus core vaccines against canine distemper and canine adenoviruses 2 provides long lasting (3-plus years) protection, including against canine adenovirus 1.
In contrast, vaccination against B bronchiseptica is considered non-core. In the UK, all currently available formulations contain attenuated live bacteria for intranasal or oral administration and mild clinical signs consistent with CIRDC can occur following their administration. These vaccines must not be administered parenterally, as inadvertent injection has been reported to result in abscessation and even death.
In the face of an outbreak they can be administered to clinically well dogs, as the onset of immunity is generally rapid. Their use should be considered in dogs anticipated to enter high-risk settings, if at all possible at least two weeks in advance.
Experimental challenge studies have indicated that both oral and intranasally administered vaccines can be effective at reducing clinical signs in the majority of dogs; however, while some suggest equivalency, others concluded that intranasally administered formulations were superior in their ability to stimulate an effective immune response10.
The benefits of intranasal administration have to be weighed up against the ability to safely administer the product, on an annual basis, particularly in light of anecdotal reports of humans developing cough following exposure to the vaccine. Owners should also be made aware that vaccinated dogs (with or without clinical signs) can shed organisms, and that this too could represent a source of infection to immunocompromised owners.
Some intranasal B bronchiseptica vaccines are combined with modified-live canine parainfluenza virus vaccines. Like B bronchiseptica, canine parainfluenza virus vaccines are considered non-core and, although they are often also included in parenteral vaccine formulations, the intranasal route of administration is considered to produce a superior immune response11.
The role of canine herpesvirus in CIRDC is controversial; however, there is a non-core vaccine available, albeit aimed at breeding bitches to reduce the risk of fading puppy syndrome. A non-core vaccine against canine influenza is available in the US, but not the UK.
PANEL 1. How to perform a blind bronchoalveolar lavage
Equipment
To perform
Potential risks