10 Oct 2016
Karen Perry discusses medical, nutritional and environmental treatment options for this orthopaedic condition.

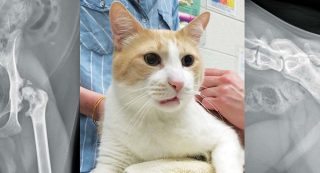
Figure 2. An approximately five-year-old male neutered Manx cat with severe left hip OA secondary to chronic craniodorsal luxation.
OA is a complex condition causing pain, inflammation and lameness in a significant proportion of small animal patients. Treatment has traditionally been directed towards palliation of the painful symptoms associated with the condition; however, this can be challenging as the goal is often variable, both within and between patients.
Monitoring of OA patients is critical to establishing a successful outcome and therapeutic protocols must be tailored to the individual patient. While NSAIDs are the most frequently recommended treatment for OA, multimodal protocols are likely to improve pain control and reduce the risk of side effects.
Treatment is likely to involve a combination of exercise modification, environmental modulation, weight management, medical management and physical rehabilitation. In patients where satisfactory quality of life cannot be achieved with this approach, surgical management should be considered.
OA is a complex condition with clinical signs including pain, inflammation and lameness. The disease is common in dogs (Figure 1) and cats (Figure 2); reports estimate up to 20% of dogs older than one year of age may develop the disease1 and the prevalence in cats has been reported to range between 16.5% and 91%2-6.

When considering the treatment of OA, a multitude of biochemical, physical and pathologic alterations must be recognised7. The decision of when and how to treat OA is often based on a combination of factors, including data available regarding efficacy, frequency of administration, product formulation, cost, personal experience and success or failure of previous treatments used by the client and patient. Treatment is further influenced by the ability or willingness of the client to understand or implement weight control, exercise modification and physical rehabilitation as part of the management strategy.
No cure for OA exists, although many different treatments are available to aid in management of the disease. Treatment of OA has traditionally been directed towards palliation of the painful symptoms associated with the condition. This can be deceptively challenging to achieve, however, as the goal is often variable, both within and between patients:
The wide range of factors affecting joint health and pain status make it difficult to provide a specific recommendation for the treatment of OA that is applicable in all situations. To achieve satisfactory relief under these extensively variable conditions, multimodal therapy is often required. Owners must be counselled from the outset that OA is likely to be a lifelong process, with flare ups that increase in severity and frequency as the patient ages8. The approach to these cases often involves a balancing act between exercise, medical management, weight management and surgery.
Although often overlooked, monitoring is a critical component of OA management. The therapeutic protocol needs to be tailored to the individual pet and only through monitoring can the clinician know what is working and when changes need to be made. Monitoring may include subjective and objective measurements, including goniometric assessment of range of motion, measurement of limb circumference to assess muscle mass, lameness scoring and force plate analysis9.
Questionnaires may be useful to assess how instigated treatments have affected quality of life10,11. Activity monitors and accelerometers can also assist with monitoring treatment efficacy12,13. Not only does monitoring assist the clinician with decision making, it can also help in keeping owners motivated, thereby maximising their compliance during management of this chronic condition.
NSAIDs are the most frequently recommended treatment for OA. NSAIDs act by inhibiting the cyclooxygenase (COX) enzymes needed to produce prostaglandins and thromboxanes. The goal of COX inhibition is to reduce the amount of inflammatory mediators, such as prostaglandin E2 (PGE2), which produce pain and inflammation by dilating blood vessels, potentiating chemical mediators of inflammation and hypersensitising central and peripheral nociceptors. However, NSAIDs used in the treatment of OA do inhibit the production of other important prostaglandins as well, which can lead to undesirable side effects.
The efficacy of NSAIDs, when compared to placebo administration, for the treatment of OA is unquestioned. Studies comparing one NSAID to another for treatment of OA in people most frequently demonstrate each NSAID is superior to placebo, but no significant differences exist between NSAIDs with respect to amelioration of symptoms14.
Although some veterinary studies suggest a difference between NSAIDs with respect to analgesic quality15, it is generally accepted little difference exists among the approved NSAIDs with respect to the level of symptom relief. However, in people, evidence exists that one NSAID may be more beneficial than another for a specific individual16. It is reasonable to presume dogs and cats have a similar response to NSAIDs and, while veterinary NSAIDs are often prescribed based on convenience of dosing, product formulation, risk of associated side effects and cost, demonstrated efficacy for an individual should be the most important.
In a patient where one NSAID does not provide symptomatic relief, this should not necessarily be interpreted as a lack of response to NSAIDs as a group and another NSAID should be trialled. The author recommends a five-day washout period between different NSAIDs to reduce the risks of attendant side effects and alternative analgesia should be offered during this time.
NSAIDs available for treatment of OA in dogs include carprofen, meloxicam, deracoxib, firocoxib, robenacoxib, phenylbutazone, mavacoxib, cimicoxib, ketoprofen and tolfenamic acid. Meloxicam is the most commonly used NSAID for treatment of OA in cats. It is licensed for long-term use and has proven to be very effective11, 17-21.
In the author’s experience, in some individual cases where meloxicam has not resulted in significant improvement, cats have subsequently responded to treatment with robenacoxib.
While it is not licensed for long-term use and can only be used for six days, it may be worth trialling in cases unresponsive to other medications. Pulse therapy can be considered for long-term management if indicated. Recommendations for prescribing practices for NSAIDs in the management of canine OA vary widely, with some recommending intermittent therapy “as required” and some recommending continuous therapy. Potential benefits of continuous therapy include better control of pain, greater improvements in mobility and the potential slowing of the disease process through improved joint usage22.
The potential adverse effects include tolerance over time to the drug, an increased incidence of adverse effects and compliance issues22. A recent review concluded evidence indicates a benefit of long-term over short-term treatment in terms of increased clinical effect and no evidence exists that this is associated with a reduction in safety22.
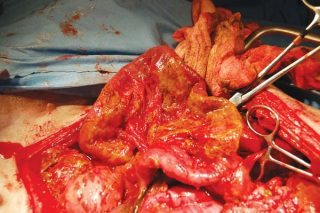
Overall, the incidence of serious adverse effects associated with NSAID use is low and it has been suggested adverse effects may have more to do with an individual animal’s inherent response to NSAIDs, rather than correlating with duration of treatment22. Having said this, COX-inhibiting drugs do carry the potential for adverse effects, including vomiting, diarrhoea, gastrointestinal ulceration and perforation (Figure 3), nephropathy and hepatopathy. It is estimated approximately 5% to 10% of patients may have to discontinue drugs because of adverse effects23 and, therefore, alternative options for pain relief are required.
Advances are being made in the pursuit of more targeted pharmacologic approaches and drugs with different modes of action, and a promising option exists in the form of grapiprant – a new analgesic and anti-inflammatory drug in the piprant class that is a potent and highly selective antagonist of the PGE2 receptor four. This was discussed in an article that appeared in Veterinary Times24.
The basis of multimodal analgesia is to use a combination of drugs that all act at different levels of the pain pathway, which will have a synergistic effect, hopefully improving pain control and possibly allowing lower doses of individual drugs to be used, thereby reducing the risk of side effects. It is likely chronic OA results in neuropathic pain in addition to the more commonly appreciated nociceptive pain and, therefore, it is probable multimodal therapy will be advantageous25. The following additional analgesics can be considered when NSAIDs do not provide sufficient relief.
Amantadine is an N-methyl-D-aspartate (NMDA) antagonist that may decrease central sensitisation and is suggested to enhance the effects of NSAIDs, gabapentin and opioids25, 26. When given in combination with an NSAID, amantadine has been proven to provide a greater treatment effect in dogs with OA than the same NSAID alone26.
Gabapentin is a structural analogue of γ-aminobutyric acid that appears to decrease central sensitisation by inhibiting presynaptic calcium channels in the dorsal horn. Given chronic OA is likely to result in neuropathic pain25, gabapentin may have a role to play in management. No studies have been published evaluating the use of gabapentin for the treatment of OA in dogs.
Pregabalin is structurally similar to gabapentin, but has higher oral bioavailability and a longer half-life23,27. Again, given the likely contribution of neuropathic pain to dogs suffering chronic OA, it may assist with management. No controlled studies exist evaluating the efficacy of pregabalin to treat neuropathic pain in dogs, although a case series of dogs with chiari-like malformation and syringomyelia reports its use28. The cost of pregabalin often prevents use as a first-line drug for veterinary patients; however, costs may reduce as the patents governing its use expire and generic pregabalin becomes available.
Tramadol is an opioid-like analgesic that acts through weak inhibition of opioid receptors, along with interference with the release and re-uptake of noradrenaline and serotonin in descending inhibitory pathways. While it has been demonstrated to be effective for treatment of OA in people29,30, no published clinical trials have demonstrated clinical efficacy for the treatment of OA in dogs.
Tramadol is metabolised into eight substrates, of which only one is active in the dog and only for a short (one to two-hour) period; if tramadol is used, careful monitoring of the patient is recommended to ensure adequate levels of analgesia have been provided31,32.
Codeine is a mu opioid agonist used for the relief of mild to moderately severe pain in humans. Codeine tablets are available as a sole ingredient or in combination with other drugs, including acetaminophen (paracetamol). Experimental studies in dogs demonstrated the antinociceptive effect of 2mg/kg codeine was similar to 0.1mg/kg morphine33.
No reports exist of oral codeine efficacy in clinical cases or controlled clinical studies, but it remains a low-cost option that can be trialled in patients with OA.
It is important to remember codeine formulations with acetaminophen will result in toxicity in cats and these are contraindicated in this species.
Amitriptyline is a tricyclic antidepressant that acts by inhibiting neuronal reuptake of norepinephrine and serotonin, thereby increasing the activity of the descending inhibitory pathways that modulate afferent nociceptive input. It also antagonises voltage-gated sodium channels and NMDA receptors25. While it has been used to treat chronic and neuropathic pain in humans, no published clinical trials exist evaluating the use of amitriptyline for treatment of OA in dogs. A small case series does describe its use, both alone and in combination with other analgesics, in three dogs for management of neuropathic pain, with mixed results34.

Steroid injection is usually reserved for cases that have become refractory to other treatments, when the animal is suffering considerably and quality of life is an issue. A long-acting intra-articular injection of methylprednisolone acetate may provide rapid alleviation of clinical signs without inducing systemic signs. The response, although rapid and significant, is often only relatively short-lived and often needs repeating once or twice. Multiple repeats in excess of this are not recommended due to the detrimental effects of the steroid on the articular cartilage. Systemic steroid therapy should be avoided due to the potential side effects of the medication.
Nutraceuticals are naturally occurring, biologically effective nutritional supplements that may confer some degree of health benefit35. Nutraceuticals may play a role in the long-term prevention of chronic disease, such as OA36. A plethora of products are available, including pentosan polysulphate, chondroitin sulphate, glucosamine hydrochloride, green-lipped mussel preparation, avocado/soybean unsaponifiables, elk velvet antler and P54FP (extract of turmeric).
The benefits of nutraceuticals in the management of OA are hotly debated. Although meta-analyses are generally not encouraging for the direct effects of these adjunctive therapies37,38, reduced use of NSAIDs has been reported in conjunction with their use39-41. The reduction in NSAID dose may help minimise the possibility of adverse effects associated with long-term administration.
While the level of evidence associated with nutraceuticals is insufficient to allow definitive endorsement of their efficacy in OA management, they are also very unlikely to be associated with significant side effects. Therefore, other than cost, no reason exists not to offer owners a trial treatment period. Most of these products take a period of time to reach a therapeutic level and, therefore, the author’s approach is to trial any nutraceutical for a period of six to eight weeks. If no improvement in clinical condition is noted after this time, longer courses of these products cannot be recommended based on existing evidence levels.
A review highlighted dietary supplementation with omega-3 fatty acids as the only nutraceutical with a sound evidence base supporting its use in companion animals38. The theory behind the use of omega-3 fatty acids is based on the fact they are incorporated into cell membrane phospholipids. Arachidonic acid (AA) is the predominant fatty acid in cell membranes; however, supplementation with increased levels of omega-3 fatty acids results in increased eicosapentaenoic acid (EPA) content42.
When eicosanoid metabolism is induced, the EPA competes with available AA as a substrate for the COX enzymes, altering the levels and even the particular inflammatory mediator produced42-44. The metabolism of EPA produces relatively fewer inflammatory prostaglandins, which can have an impact on the catabolic pathways and, therefore, on disease progression in OA36.
Ingestion of omega-3 fatty acids may also reduce the serum concentrations and activities of proteoglycan-degrading enzymes, COX-2 and inflammation-inducible cytokines45. Dietary supplementation with fish oil omega-3 fatty acids increases blood concentrations of these fatty acids and has an ameliorative effect on OA in pet dogs, according to owners’ assessments46.
Supplementation has also been shown to result in improved weight bearing in dogs with OA47 and to allow for a more rapid reduction in carprofen dosage compared with feeding a controlled diet40. One study in cats also demonstrated an increase in activity levels in cats fed an omega-3-rich diet, based on owner assessment48.
Mlacnik et al53 demonstrated a combination of caloric restriction and physiotherapy led to improvements in patient mobility and facilitated weight loss. Kealy et al50 reported the prevalence and severity of OA were less in dogs with long-term reduced food intake and that this also delayed the onset of signs of chronic disease and increased the lifespan of these dogs51.
Evidence also exists of improved quality of life when obese dogs lose weight and this includes significant improvements in activity and evidence of a decrease in chronic pain. Many owners struggle with weight control in their pets and it is important to emphasise to them the benefits of improved mobility can be seen with only modest weight loss; improvements in gait become evident on force plate analysis once bodyweight loss exceeds 6%52. This weight loss can be achieved within two to three months and at least 80% of dogs will be successful with such a programme55.
Weight control is an integral aspect of managing OA in dogs, and weight loss alone may substantially improve clinical signs in overweight dogs with OA.
Very little is known about the effects of exercise on OA. In people, it is generally recommended some exercise is beneficial and, in veterinary patients, while exercise should be moderated, it should not be stopped entirely. Anecdotally, no exercise may be as detrimental as extremes of exercise8.
High-impact activities should certainly be avoided, such as ball or frisbee play or agility, but consistent levels of low-impact activity should continue. When a flare up of OA occurs, exercise is generally reduced to short lead walks several times a day, which are gradually increased over a period of several weeks, if appropriate.
Environmental modulation may be important for OA management in cats. The pain associated with feline OA seems to cause multiple significant behavioural changes in cats and modifying the environment, so as to allow the cat to resume as much normal behaviour as possible, will improve both its physical and psychological well-being56,57.
Specific environmental aspects to consider include:
Physical rehabilitation is the treatment of diseases and injuries with physical agents such as heat, cold, water, sound, electricity, massage and exercise58. Its benefits may result from increasing blood and lymph flow through the affected area, resolving inflammation, preventing or minimising muscle atrophy, preventing periarticular contraction and providing positive psychologic effects for the patient and owner58.
A paucity of scientific literature exists documenting the effectiveness of physical rehabilitation techniques in small animals, with much being extrapolated from the human literature. However, interest and research in this field is increasing and rehabilitation may be used in patients with OA to reduce pain, control inflammation, improve strength and balance, increase range of motion, prevent muscle spasms and help restore more normal joint function9, 59-64.
In many cases, physiotherapy also helps to reduce the dose of analgesics necessary to maintain patient comfort64.
Rehabilitation programmes for patients with OA generally consist of a combination of modalities, which may include cryotherapy, moist heat, passive range of motion exercises, stretching exercises, balance and proprioception exercises, massage therapy, therapeutic ultrasound, laser, electrical stimulation and active exercise (Figure 4).
Rehabilitation programmes must be customised for individual patients and should be designed to encourage increased weight bearing, enlarged muscle mass and reduced body fat, thereby breaking the cycle of disuse often seen in patients that have OA9,62,64.
Research is progressing in the field of cellular therapy as a therapeutic option for dogs with OA-related pain that proves refractory to other treatments. Among these therapies are autologous platelet therapy and mesenchymal stem cells. These were discussed in an article that appeared in Veterinary Times65 and will not be discussed in detail here. While more research is indicated in these areas, some evidence exists to support their use.
Most patients with OA can be managed with a combination of non-surgical therapies; however, for those where a satisfactory response is not achieved, surgical management should be considered. The mainstay of surgical OA management in the veterinary field is total joint replacement. Commercial joint replacements are readily available for the hip, stifle and elbow in the dog and for the hip in the cat, while customised joint replacements have also been performed for other joints, including the canine ankle and shoulder, and the feline stifle.
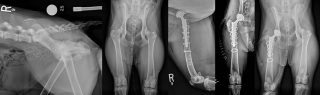
Of these, total hip replacement is the most commonly performed and has the most consistently successful results with excellent function being achieved in more than 90% of cases66. Due to these results, total hip replacement is often performed at a much earlier stage than previously recommended. As long as a patient has reached skeletal maturity and experiences discomfort associated with OA on a frequent basis, they can be considered candidates for total hip replacement and many of these patients go on to lead normal lives, with some returning to agility or work (Figure 5).
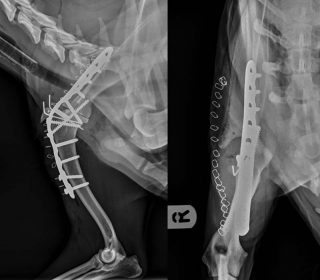
Stifle – and particularly elbow – replacement surgeries, however, remain technically much more challenging with higher complication rates associated and these remain salvage procedures, only appropriate once all other management regimes have failed. It must be remembered, in a high percentage of cases, OA affects multiple joints. Therefore, even following a successful total joint replacement, ongoing management of disease in other joints may still be required although replacement of the most severely affected joint should certainly facilitate this.
In cases where total joint replacement is not feasible, arthrodesis may be a suitable alternative. Arthrodesis relieves the pain associated with end-stage OA, but obliterates motion at the joint and is, therefore, better suited to lower motion joints, such as the carpus and tarsus. Shoulder arthrodesis is well tolerated, but can be technically challenging (Figure 6).
Arthrodesis of the stifle – and particularly the elbow – result in profound gait abnormalities and careful case selection and client communication are critical in these cases. In the author’s experience, mobility following amputation may be better than that following elbow arthrodesis, as long as amputation is not precluded by severe disease in other joints. Arthrodesis of the hip is not possible and, in cases where total hip replacement is not an option, femoral head and neck excision is the salvage surgery of choice.