21 Nov 2016
Paul Burr and Danièlle Gunn-Moore look at the zoonotic potential of some members of this pathogen group, as well as the complexities in diagnosing infection and tests available in the UK.
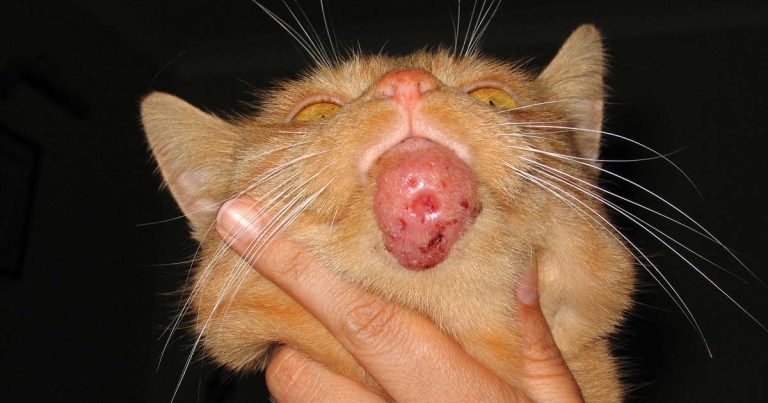
Figure 1. A chin lesion caused by Mycobacterium bovis.
Mycobacterial disease is a key challenge facing the veterinary profession throughout the UK. Bovine tuberculosis (TB) in cattle and badgers receives the most attention, but spillover cases in cats in the Newbury area raised the profile of TB in companion animals and other species.
Diagnosing feline mycobacterial disease is complex and, given concerns over the zoonotic potential of the tuberculous group of mycobacteria, a general diagnosis of mycobacteriosis is perhaps no longer sufficient; it is important to obtain an indication of the class of Mycobacterium involved and, hence, the risk to other household members.
As is the case for most infectious diseases, diagnosis relies on a combination of clinical signs, history and appropriate use of diagnostic tests. Tests available in the UK, their use and limitations are reviewed.
Mycobacteria comprise a large group of morphologically similar bacteria. The group has shared features, such as a high lipid content cell wall (acid-fast), resistance to heat, pH change and disinfectants.

Clinical classification relies on culture characteristics – impossible, slow-growing or fast-growing – and the tendency to produce granulomatous disease with or without dissemination or tubercles. Tubercles are defined as small, round, translucent lesions with central caseation and surrounding granulomatous inflammation, and can be associated with zoonotic potential.
The features used in classification are generally supported by genetic classification and provide clues as to the unique challenges associated with the diagnosis and control of mycobacterial infection.
Some mycobacteria of veterinary importance are listed in Table 1.
In general terms, the tuberculous complex has the most zoonotic potential and tendency to disseminate within the host. However, even here, infection may be latent, or disease may be subclinical, and exposure to infection does not always result in disease.
The opportunistic, slow-growing group (Mycobacterium avium complex) are sometimes localised to cutaneous lesions, but can also disseminate in immunocompromised hosts. A member of this group causes Johne’s disease in cattle.
Opportunistic, fast-growing mycobacteria generally cause localised cutaneous and subcutaneous granulomas following bite or puncture wounds.
Feline leprosy refers to single or multiple granulomas in the skin or subcutis caused by Mycobacterium lepraemurium or another, as yet unidentified, species.
It has been reported 1% of biopsy samples have a suspicion of mycobacterial disease in UK cats. It could be argued infection is a differential in many chronic respiratory, alimentary or cutaneous problems.
Diagnosing feline mycobacterial disease, as for most infectious diseases, relies on a combination of clinical signs, history and appropriate use of diagnostic tests. Specific diagnostic techniques for all infectious diseases rely on either detection of the organism or the host’s response to it.

To use tests properly, it is critical to understand the disease process and that the presence of a pathogen, or an immune response to the pathogen, does not prove the pathogen is the cause of the disease being investigated. Results must be interpreted in the context of what is known about the disease pathogenesis and clinical signs.
For mycobacterial infections, interpretation must take into account the genetics of the pathogen, and host genetics/immune response will influence disease progression. Animals may also be exposed to mycobacteria and harbour infection without developing clinical disease.
Detection of acid-fast bacteria from cytology or histopathology is a useful starting point, but confirmed cases may not have detectable bacteria by these methods.
Culture, from fresh tissue, was historically regarded as the reference diagnostic technique. However, this is not without difficulty as mycobacteria can be slow, difficult or impossible to grow. Culture is a specialist technique dependent on the skill of the laboratory and culture conditions designed to favour one species may inhibit the growth of another.

PCR-based testing – most often of tissue biopsies or cytology slides where acid-fast bacteria have been identified, or even as a follow-up from early culture in some circumstances – has the potential to rapidly discriminate certain classes of mycobacteria. However, tests available in the UK are for Mycobacterium tuberculosis complex and Mycobacterium avium complex bacteria. The test discriminates between these groups, but not within them – for example, Mycobacterium bovis versus Mycobacterium microti infection cannot be differentiated at present.
PCR is not infallible and, like other techniques, may struggle if very low numbers of mycobacteria are present.
Tests to detect the host response to mycobacteria are widely used in farm animals. Antibody tests are used in Johne’s disease, and intradermal tuberculin testing and interferon gamma release assay (IGRA) testing are at the forefront of TB testing in cattle. Antibody tests are available, but controversial.
In companion animals, although research is ongoing into the use of antibody tests, none are widely used. Published results suggest antibody-based tests have good specificity, but poor sensitivity, particularly for M microti infections. Tuberculin testing is not thought to be useful in cats and not often used in dogs.
Following cultivation, supernatant is collected from each well and tested for interferon gamma in an ELISA (Figure 4). Depending on the precise interpretation criteria used, this method in cats has shown sensitivity of 70% to 100% and specificity of 95% to 100%.
Testing of in-contact animals in a multi-pet household, and testing to monitor treatment, has also been proposed.
Diagnosis of many infectious diseases relies on careful evaluation of the clinical signs and the available diagnostic tests. Mycobacterial disease falls into this category and is of particular importance because of the zoonotic potential of some members of the group.
Follow-up testing to determine the species involved and zoonosis risk is advisable when strong grounds exist to suspect mycobacterial infection.