27 Oct 2015
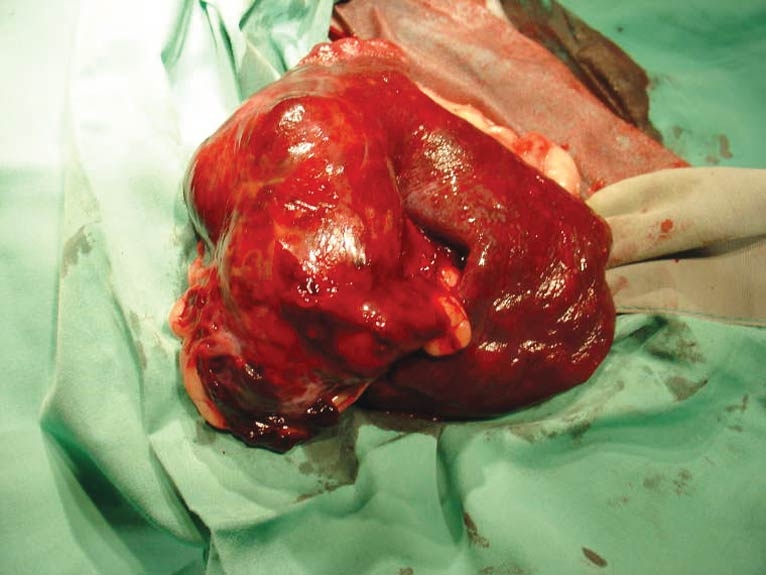
Figure 1. Spleen with haemangiosarcoma post-splenectomy. A provisional study suggests metronomic chemotherapy may have a role in improving survival compared to surgery alone.
In the past few years, several exciting drug developments have been specifically marketed for treatment of neoplastic disease in veterinary patients.
These have largely arisen due to an increased understanding of the science behind cancer development and treatment, such as the underlying role of mutations/dysregulation of the c-kit gene in mast cell tumours. There have also been some alterations in the way we schedule traditional medications to exploit certain biological processes such as tumour blood vessel formation and growth (for example, metronomic chemotherapy).
The purpose of this article is to provide a summary of some of these newer, emerging treatments.
The goal of traditional chemotherapy has been to administer as much drug as can be tolerated, with the notable caveat that veterinarians and their clients are generally less willing to accept a high degree of side effects, which most often results in relatively lower drug doses than are used in human oncology.
Still, whether toxicity is expected, the resulting schedule requires a break period of sufficient duration to allow susceptible normal tissues, such as the gut and bone marrow precursor cells, to recover before the next dose can be given. This break period can be as long as two to four weeks depending on the chemotherapy agent. Examples would be carboplatin for osteosarcoma and doxorubicin or epirubicin for haemangiosarcoma.
Although this approach has resulted in good responses, and even cure for certain cancers, it is increasingly acknowledged gains made in the treatment of a number of solid tumours with conventional chemotherapy may have reached a plateau. Future success depends on newer approaches.
Conventionally, the goal has been to raise the dose to the highest level tolerated by the patient, the so-called maximum tolerated dose (MTD). This is based on previous demonstration that logarithmic tumour cell kill occurs with increasing chemotherapeutic dose (the bigger the dose of drug, the bigger the tumour cell kill).
This may not be applicable to the majority of solid tumours that exist within a complex microenvironment. By providing continuous treatment, tumour cells may be more continuously exposed to the drug, which would permit more efficient cell kill as cells continue to cycle.
For solid tumours to grow beyond a few millimetres, a network of vessels must be recruited to bring oxygen and nutrients. This is known as angiogenesis.
Firstly, there is a direct cytotoxic effect on the endothelial cells of the blood vessels. Secondly, there appears to be an indirect effect by altering the relative balance of angiogenic growth factors and inhibitors preventing further vessel expansion. Finally, the expansion of tumour blood vessels may also require, or at least be influenced by, recruitment of bone marrow-derived endothelial progenitor cells.
Previously, it was thought all new endothelial cells were derived from local division of differentiated endothelial cells in pre-existing vessels. However, circulating endothelial progenitor cells (CEPs) can be mobilised from the bone marrow to sites of ongoing angiogenesis. Such cells can be mobilised out of the bone marrow in response to a number of pro-angiogenic factors, and therefore are also considered a target of antiangiogenic treatment strategies.
There appears to be a lack of perturbation of normal physiologic angiogenesis, as occurs in wound healing. In addition, inhibition of proliferation and migration of endothelial cells at minute drug concentrations has been demonstrated. This is attractive for perioperative use of metronomic schedules.
Tumour cell destruction may be mediated by lymphocyte responses including B and T cells. However, these types of immune responses may be stunted by a subset of lymphocytes known as regulatory T-cells (Tregs), which are normally involved in autoimmune disease prevention as well as normal immune tolerance. Metronomic administration of certain chemotherapy agents has been shown to decrease Treg number and proportion in dogs with cancer; thereby enhancing the immune response.

When compared with historical controls treated with surgery alone, there was a significant prolongation of the disease-free interval in the chemotherapy-treated dogs. This may represent a possible therapeutic option in cases where radiotherapy is not indicated, declined or not possible (Elmslie et al, 2008).
Both studies reported acceptable toxicity associated with the protocol. One concern regarding the use of cyclophosphamide is the possibility of sterile haemorrhagic cystitis, which is a known potential toxicity with the use of this drug in dogs. Up to 10 per cent of dogs had evidence of this side effect at some point during treatment (Elmslie et al, 2008).
The results of these veterinary studies suggest, like many therapies that target the growing tumour vasculature, objective results with metronomic chemotherapy may take considerable time to develop and may only manifest as sustained stable disease. For instance, it is not unusual for actual tumour shrinkage, if it occurs, to manifest only after one to two months of continuous therapy, or more. Many patients achieve only stable disease or a slowing of disease progression.
In addition, like many applications of chemotherapy for solid tumours, the benefits of metronomic dosing may be maximised at the lowest tumour burden – that is, as adjuvant therapy as opposed to being given when a large tumour burden is present.
Based on the initial studies, dosing was described as:
A study (Burton et al, 2011) suggested a larger reduction in Tregs and more evidence of antiangiogenesis occurred on 15mg/m2 daily and that slightly higher doses might be explored in future clinical studies.
Regular haematological monitoring (at least initially) is advisable and weekly dipstick testing of the urine should be considered to identify any haematuria and ensure owners are closely monitoring urination. The owners can do this at home and should remain vigilant in looking for signs of developing cystitis, even in the long-term.
It is important to keep in mind antiangiogenic therapies and metronomic scheduling, on their own, may not replace more traditional, intensive cytotoxic schedules. Perhaps these approaches may ultimately be used in combination although this requires further evaluation in the veterinary literature. Provisional studies suggest combination of MTD chemotherapy and metronomic chemotherapy is both feasible and safe in dogs.
Research into the effects of metronomic chemotherapy continues to focus on understanding its effects on angiogenesis, immunology and other components of the tumour microenvironment, as well as tumour cell specificity and the nature of drug-
resistance mechanisms that emerge during treatment.
Clinical trial evaluation of low-dose, continuous chemotherapy regimens continues in both human and veterinary oncology, and, therefore, despite the appealing nature of the convenience and cost of this approach, metronomic chemotherapy protocols are still considered investigational and not the current standard of care.
Thalidomide has been used in cancer therapy for a long time, since it initially ceased to be used due to its well-known potent teratogenic properties.
In human patients lenalidomide appears to be more commonly used. Lenalidomide is a thalidomide analogue and is a standard agent in the treatment of multiple myeloma and myelodysplastic syndrome, but appears to have modest antitumour effects across a broad range of malignancies. The mechanism of action of these drugs is uncertain, but includes inhibition of vascular endothelial growth factor, which is involved in tumour blood vessel formation, and inhibition of multiple tumour-promoting and inflammatory cytokines including interleukin (IL)-1b, IL-12 and IL-6, which are associated with myeloma cell survival.
Thalidomide has shown some activity in canine haemangiosarcoma, in vitro in osteosarcoma and, anecdotally, in some solid tumours; presumed to be due to its antiangiogenic properties.
The drug is fairly expensive and there are clearly some health and safety issues with owners handling it. However, it appears to be well tolerated with few side effects noted in dogs and, anecdotally, can be combined with other agents.
Lenalidomide appears to be cost-prohibitive in veterinary patients.
Some tyrosine kinases are intricately involved in growth and progression of the tumour-associated vasculature, such as vascular endothelial growth factor receptor and platelet-derived growth factor receptors. These drugs can therefore exert a useful antiangiogenic action and will be discussed in part two of this article.