9 Nov 2015

The first part of this article on new or updated treatments for veterinary patients with cancer discussed the possibility of targeting the tumour-associated vasculature with treatments such as metronomic chemotherapy, thalidomide and an introduction to tyrosine kinase inhibitors (TKIs).
This part will expand on TKIs and discuss some exciting immunological treatments, such as vaccination strategies for canine melanoma, lymphoma and feline sarcoma. Some treatments on the horizon also have a high chance at significantly extending the survival of canine lymphoma patients.
Work has implicated the tyrosine kinase, known as KIT, in the aetiology of canine mast cell tumours (MCTs). KIT is a membrane-surface growth factor receptor expressed on mast cells (MCs) and encoded by the proto-oncogene c-kit. KIT is activated when the external receptor is bound by external growth factor. Activated KIT initiates a signalling cascade that culminates in proliferation, migration, maturation and survival of mast cells. KIT expression has been demonstrated in both normal and neoplastic canine MCs, with higher expression in poorly differentiated (high grade) MCTs. Studies have shown about 15% to 40% of all canine MCTs are affected by c-kit mutations, which thus encodes a faulty KIT protein.
In addition, a significant association between mutations and tumour grade has been demonstrated (3% of well-differentiated,
28% of intermediate grade and 41% of high-grade tumours, respectively, were affected; Thompson et al, 2015).
Mutations of c-kit produce an abnormal, constitutively activated KIT protein in the absence of external receptor binding – meaning increased proliferation, survival and growth of affected cells.
The role of these receptor tyrosine kinases in canine MCTs is important in understanding the role and mechanisms of the newer drugs that have recently been marketed for canine MCTs: the TKIs.
TKIs can bind to and inactivate tyrosine kinases, as described previously, which are involved in cell growth, proliferation and blood vessel formation. They potentially have anti-cancer activity and can also be antiangiogenic (prevent the formation of blood vessels that are important for tumour growth). Therefore, these drugs can kill neoplastic cells by direct and indirect mechanisms.
Two veterinary TKIs are available for use in canine cutaneous, non-resectable grade two and three mast cell tumours: toceranib phosphate (Palladia; Pfizer) and masitinib (Masivet; AB Science).

Treatment candidates are:
The dose of masitinib is as on the data sheet. The labelled dose for toceranib is 3.25mg/kg on alternate days. However, a significant number of dogs have (in the author’s experience) unacceptable toxicity at this dose, which requires dose reduction. The author therefore prefers to start dogs on 2.4mg/kg to 2.8mg/kg every Monday-Wednesday-Friday, which seems to be a common approach among oncologists in the UK.
Prednisolone can be used alongside toceranib and other TKIs. Gut protectant drugs are advised. Dogs treated with toceranib with MCT typically have gross (macroscopic) disease, therefore the author considers gut protectants (such as omeprazole) imperative during treatment.
Response rates of around 40% to 50% can be anticipated in canine MCTs. Median response duration is around 4 months to 4.5 months in toceranib dogs, based on the initial studies (Miller et al, 2014; London et al, 2009). This is a median, so some dogs only have transient responses and some respond for much longer periods. The author has seen a number of dogs that have had complete and rapid responses to toceranib, which have lasted for months to years – even in the presence of gross metastatic disease. Response is clearly highly variable. Unfortunately, in most cases drug resistance occurs resulting in disease progression.
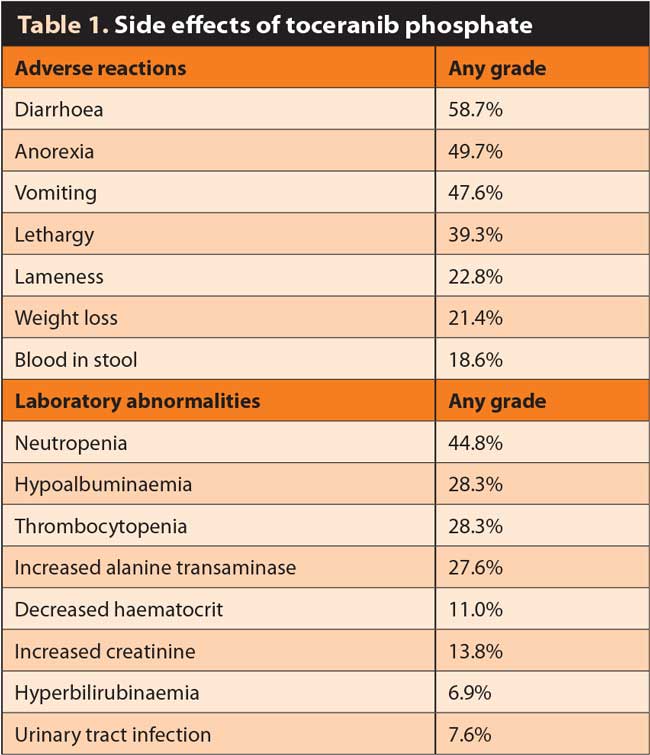
As can be seen in Table 1, the side effects of toceranib are not benign or rare. The adverse effects of masitinib are broadly similar. However, these drugs were based on the clinical trial where the labelled dose was 3.25mg/kg (higher than is typically clinically used now). Therefore, this may be taken as a maximum and the author has found while gastrointestinal (GI) effects in particular are reasonably common even at the lower dose, the majority are very minor and can be managed easily.
The data sheets for toceranib and masitinib have details of how dose reductions/holidays should be performed for various side effects. Typically, this is cessation of the drug for up to a week (depending on the clinical context and severity of the reaction and often reinstitution with a dose reduction; again based on severity or owner concerns).
In traditional chemotherapy, neutropenia can be potentially marked and clinically significant (depending on the drug), but with TKIs neutropenia is typically mild, doesn’t warrant antibiotics and rarely requires a significant dose reduction. The neutropenia tends to be in the 2.0 × 109/l range.
It is important to remember response rates are often not durable and typically last only a short number of weeks to months. Also, although these drugs are not “conventional chemotherapy” drugs, one should remember they do have a high incidence of similar side effects to chemotherapeutics, including anorexia, GI upsets, haematological dyscrasias and renal disease, to name but a few.
Very careful monitoring is required during treatment and owners should be counselled appropriately.
A significant number of cutaneous feline MCTs also appear to harbour c-kit mutation and thus may be candidates for TKI therapy. For most patients this is not a major consideration as many feline MCTs are benign in behaviour and surgery is curative.
However, in cases with a more aggressive clinical course or multiplicity of tumours, TKI therapy may be appropriate, although case selection should be guided by an oncologist because of the relative rarity of this clinical situation. Masitinib has been shown to be safe in cats and, anecdotally, the author has found toceranib very well tolerated in cats.
Splenic and intestinal MCTs in cats can have an aggressive clinical course, though they appear to rarely harbour c-kit mutations. TKI therapy is likely to be disappointing in these cases.
Due to their antiangiogenic and antiproliferative/survival activity, TKIs are also used extensively in human medicine for solid tumours such as breast, lung and melanoma. In many of these tumours where these drugs are used, there are mutations or over-expression in particular tyrosine kinases such as HER2 or epidermal growth factor receptor (EGFR).
In canine and feline medicine, TKIs are becoming more widely used for non-MCT cancers where there are no good standard options for therapy or those patients have already failed standard treatments (recurrence or metastasis). In theory, as these drugs have antiangiogenic activity (and all solid cancers need to establish and grow their own blood supply to grow beyond a few millimetres) any histologic type could be treated with these agents.
In a study evaluating the TKI toceranib phosphate in dogs with advanced solid malignancies, it is worth noting dogs with thyroid carcinoma and anal sac apocrine gland adenocarcinoma had modest response rates (including 25% partial response in dogs with anal sac carcinoma, with a significant number achieving stable disease; London et al, 2012).
The author uses a combination of both metronomic chemotherapy and TKI modalities for non-MCT cancers if feasible for management of advanced local tumours, or in cases of metastatic disease for which animals have failed standard therapy or there are no good alternatives.
This can also be used where owners, after careful discussion, have discounted more aggressive or standard therapy.
The author starts with cyclophosphamide (10mg/m2 to 15mg/m2 daily) with firocoxib at standard doses (the metronomic chemotherapy). If, after one to three weeks there have been no significant adverse effects then toceranib phosphate will be added at a dose of around 2.4mg/kg to 2.8mg/kg every Monday, Wednesday and Friday.
Strict haematological and biochemical monitoring is mandatory (fortnightly initially and eventually monthly).
This approach appears to be very safe, and staggering the protocols ensures any adverse effects from one of them is more obvious. The author has found this approach very tolerable for the patients, with few owners wanting to cease therapy due to adverse events. Diarrhoea and other signs of GI upset can be a frustrating feature in some patients, although this can typically be well managed and corrected by a toceranib dose holiday and reinstitution at a lower dose.
Tumour vaccination holds promise for the treatment of cancer in humans and companion animals. The immune system can control tumour growth and metastasis via immunosurveillance. This approach in cancer therapy has two potential advantages – it has no or only minor adverse effects and can result in a memory that protects the patient from relapses.
Recently, Oncept, a therapeutic tumour vaccine, was approved for the treatment of oral, mucosal melanoma in dogs. Recent insights into how the immune response is suppressed in cancer patients and how it can be modulated to enhance vaccination efficacy will contribute to further improvement of vaccination strategies.
Tumour vaccination still has some weaknesses, however. Vaccines do not have an immediate effect on tumour growth and it takes a long time to develop an effective anti-tumour immune response and even more time before it is translated into a clinical response. Highly aggressive tumours (including canine oral melanoma) can cause patient death before benefits from vaccination could be expected.
Also, cancer can become resistant to immunotherapy via varying mechanisms, such as loss of major histocompatibility complex molecules or tumour antigens, production of soluble immunosuppressive factors and accumulation of suppressive cells, such as regulatory T-lymphocytes
and myeloid-derived suppressor cells.
Oncept is a DNA vaccine encoding the human tyrosinase gene. US licensing followed after a successful clinical trial that resulted in prolonged survival compared with historical control dogs.
It is administered transdermally via a needle-free device every fortnight for four treatments in total (induction course). Thereafter, a single booster injection is given every six months.
Early preliminary trials with a heterogeneous patient population of 170 dogs suggested longer survival times than commonly found for oral melanoma. A total of 75% of vaccinated dogs are expected to live beyond 464 days versus 156 days for the control group (Grosenbaugh et al, 2011). To definitively prove the efficacy of Oncept a randomised, placebo-controlled study should be performed; particularly given some smaller recent studies, which report no overt benefit in dogs receiving vaccination in addition to local treatment of the primary tumour (that is, surgery/radiation).
Currently, based on the evidence available and the absence of other successful therapies, the author recommends Oncept vaccination to all dogs with locally controlled oral melanoma – particularly given the absence of any significant adverse effects. There is some data to suggest dogs with digit (nailbed) melanoma may also derive benefit from vaccination.
The author also offers Oncept vaccination to all dogs with histologically malignant melanoma regardless of anatomic site, because the vaccine target (tyrosinase) is present in these tissues also. Readers should note the vaccine can only be administered by oncology specialists and requires a special treatment certificate to be obtained from the VMD on a named patient basis (acquired by the treating oncologist).
Oncept IL-2 contains feline interleukin-2 recombinant canarypox virus. It is used to treat cats with fibrosarcoma in combination with surgery and radiotherapy to reduce the risk of/delay the tumour recurring locally. It is recommended for use when the size of the tumour is between 2cm and 5cm in diameter and there is no evidence of metastatic disease.
Treatment begins the day before radiotherapy and preferably within one month of the removal of the tumour by surgery. A course of treatment consists of six doses of 1.0ml, the first four given at intervals of one week, and the last two at two-week intervals. Each 1.0ml dose is divided into five injections (of approximately 0.2ml each) administered under the skin around the site of the surgically removed tumour.
The active substance in Oncept IL-2 is a “carrier” canarypox virus that contains the gene to produce feline interleukin-2 (IL-2). This works by stimulating T lymphocytes to attack residual cancer cells. The virus allows the production of IL-2 in small amounts and for a prolonged time at the injection site. Canarypox viruses do not spread or multiply in cats.
Oncept IL-2 was studied in two unpublished field studies involving 71 cats that had spontaneously developed, non-metastatic fibrosarcoma. The two studies compared a total of 48 cats treated with Oncept IL-2 with 23 control cats that just received surgery and radiotherapy. The studies showed in cats treated with Oncept IL-2 the tumours took longer to come back (more than 730 days) compared with control cats (287 days).
Treatment reduced the risk of relapse in the period from six months after the start of treatment by approximately 56% after one year and 65% after two years.
Side effects that may be seen are short-lived apathy and hyperthermia. A moderate reaction at the injection site may occur that involves signs of pain when the area is touched, swelling and scratching. It usually disappears spontaneously within one week.
It is important to note the best outcomes in cats with feline injection site sarcoma have been achieved with initial aggressive surgery by a board-certified specialist surgeon, most recently with 5cm margins. This treatment should not be seen as an alternative to this modality.
Canine lymphoma is a significant veterinary problem in the UK, and the majority of canine lymphomas are of B-cell origin.
The current standard of care is multi-agent CHOP chemotherapy (cyclophosphamide, vincristine, doxorubicin and prednisolone). Unfortunately, this is rarely curative and so it is still has a high mortality rate despite initially excellent therapeutic responses.

The creation of the monoclonal antibody rituximab revolutionised human B-cell lymphoma treatment by significantly increasing response and survival rates compared to chemotherapy alone. The standard of care for human B-cell lymphoma is now rituximab in combination with CHOP. The target of rituximab is CD20, a protein expressed on B-cell lymphoma cells (but not T-cells). Rituximab is a chimeric antibody and works by depleting CD20-positive B-cells, largely via antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Rituximab does not bind to canine CD20, making it impossible to use rituximab for treatment of canine BCL (Figure 2).
A trial is under way in the US of a canine rituximab and provisional results show an improvement in outcome when combined with CHOP chemotherapy compared to CHOP chemotherapy alone. Hopefully, this will become commercially available after further toxicity and outcome data are available.
Some human T-cell lymphomas express the membrane protein CD52, which can be targeted by the anti-CD52 antibody alemtuzumab. This has led to some success in the treatment of patients with a variety of T-cell lymphomas, including some cutaneous forms, but mainly specific subtypes of peripheral, nodal T-cell lymphoma.
A canine anti-CD52 antibody is in development and trials and further information on toxicity, response and outcome is eagerly awaited – particularly because T-cell lymphomas are often less chemotherapy-responsive, with shorter survival times.
For dogs with lymphoma, particularly while monoclonal antibodies are unavailable, other immunological approaches to treatment
have been shown to be effective in increasing remission and survival times in addition to traditional chemotherapy approaches.
One promising method is the delivery of an autologous vaccine consisting of hydroxyapatite ceramic powder and proteins purified from the patients’ own individual tumour, such as heat shock proteins (HSPs).
HSPs are synthesised under stress situations (including cancer) to protect the cells from damage and play a key role in bringing tumour-associated antigens to professional antigen-presenting cells, thereby leading to immune stimulation.
HSPs bind the small proteins and peptides they chaperone, forming HSP-peptide complexes, providing a fingerprint of the tumour peptides, both normal and abnormal. Therefore, if purified from the patient’s own tumour cells, HSPs may enhance the patient’s immunity by inducing specific and non-specific cellular immune responses.
In dogs with B-cell lymphoma some provisional studies show improved outcome when combined with traditional CHOP chemotherapy and the safety and tolerability is excellent (Marconato et al, 2015). The patient is required to undergo a lymph node biopsy to enable creation of the vaccine, which is performed in-house using a specialised biochemical technique to extract tumour-associated antigens and HSPs, using freshly frozen tissue. The injections are given over several weeks, administered concurrently with chemotherapy.
Unfortunately, due to adverse effects in dogs with T-cell lymphomas, the vaccine is not suitable for this group of patients.
This technique, given all tumours possess antigens capable of immune stimulation, is not limited simply to B-cell lymphoma. Other tumours, including high-grade/aggressive solid cancers with an anticipated high metastatic rate, but with no suitable/viable chemotherapy options, may also benefit – and cases should be discussed with an oncologist if referral is sought.
This technique is, to the author’s knowledge, only being performed in the UK at Willows Referral Service in Solihull. Suitable cases should be discussed with a member of the oncology team before referral.
New drugs are available for treating animals with cancer in the UK and are widely used, and more are on the horizon. The general practitioner should become familiar with these treatment strategies to be able to advise owners about the best and most advanced therapeutic options.