13 Aug 2018
Marge Chandler considers the role of nutrition and supplements to manage this condition, in addition to addressing patient stress.

Image © chendongshan / Adobe Stock
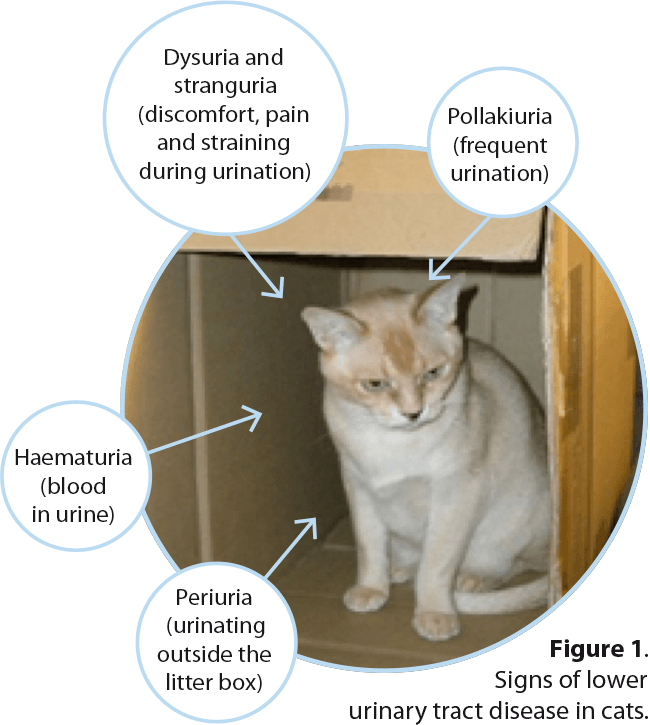 The lower urinary tract comprises the bladder, urethra and, in the male, prostate. Many lower urinary tract diseases (LUTDs) share common clinical signs regardless of cause; it is usually not possible to determine specific aetiologies from the history and clinical signs alone.
The lower urinary tract comprises the bladder, urethra and, in the male, prostate. Many lower urinary tract diseases (LUTDs) share common clinical signs regardless of cause; it is usually not possible to determine specific aetiologies from the history and clinical signs alone.
Dysuria, stranguria and pollakiuria almost invariably localise the disease to the lower urinary tract and, along with haematuria, are the common signs associated with LUTD. In cats, urinating outside the litter box (periuria) can be a manifestation of cystitis (Figure 1).
LUTD may not affect the patient’s general health and does not cause polyuria, polydipsia or azotaemia. Disorders of the lower urinary tract (excluding the prostate) include infectious and sterile cystitis, feline idiopathic/interstitial cystitis (FIC), urolithiasis/plugs and neoplasia (Panel 1).
Bacterial cystitis and FIC will be addressed in this article, focusing on management, nutrition and supplements.
Many causes of cystitis exist. The most commonly seen (with uncommon causes in parentheses) are:
Bacterial cystitis is generally uncomplicated and most commonly seen in older female dogs. Most uncomplicated bacterial urinary tract infections (UTIs) can be successfully treated with an antibiotic chosen based on culture and sensitivity, used for an adequate duration.
Complicated UTIs relapse with the same bacteria or recur with a different one. These can be caused by inadequate antibiotic duration or bacteria in an occult location (for example, a prostatic cyst) or in a protected bacterial biofilm. Antibiotic resistance is also common with urinary bacterial pathogens.
Complicated bacterial cystitis can also occur due to predisposing factors, such as anatomical abnormalities (ectopic ureters, recessed vulva and obesity) or systemic disease (for example, diabetes mellitus or hyperadrenocorticism), which decrease urine concentration and immune function.
In more than 25 per cent of dogs with recurrent UTIs, a cause cannot be identified, so treatment to prevent infection may be warranted (Wood, 2017). One treatment adopted from use in humans is cranberry juice or extract, although a Cochrane review of 24 studies concluded the evidence for preventing UTIs in humans is small (Jepson et al, 2012; Figure 2).
Previously, it was thought the urinary acidifying effect of cranberry juice was antibacterial; however, cranberry juice does not acidify the urine sufficiently to have this effect (Bartges, 2010).

A study of cranberry extract showed decreased in vitro adherence of Escherichia coli to Madin-Darby canine kidney cells (Chou et al, 2016). An antibacterial effect may occur from proanthocyanidins with A-type linkages – an active compound in cranberries that inhibits the binding of P-fimbriae of uropathogens to mannose-like residues on uroepithelial cells (Olby et al, 2017).
In dogs with spinal cord injury – at increased risk of developing bacteriuria due to increased residual urine volume – cranberry extract had no benefit compared to placebo in reducing bacteriuria (Olby et al, 2017). The study results may have been impacted by low numbers (low statistical power), therefore more studies are needed to prove the efficacy of cranberry juice/extract on preventing UTIs.
The use of D-mannose has been suggested to decrease bacterial adherence by blocking the ability of lectins on type one fimbriae to interact with uroepithelial cells. A study in women showed some reduction in reinfection, but evidence in dogs and cats is lacking (Kranjčec et al, 2014).
Glycosaminoglycan (GAG) therapy could decrease bacterial-induced uroepithelial injury by improving the health of the GAG layer in the bladder. Instillation of hyaluronic acid decreased UTI recurrence in humans, but no clinical evidence exists in dogs and cats (Wood, 2017).
In summary, no supplements have been proven to decrease the risk of recurrent UTIs; however, studies carried out have been small or non-existent, so it is possible these therapies may provide some benefit although it is likely to be small.
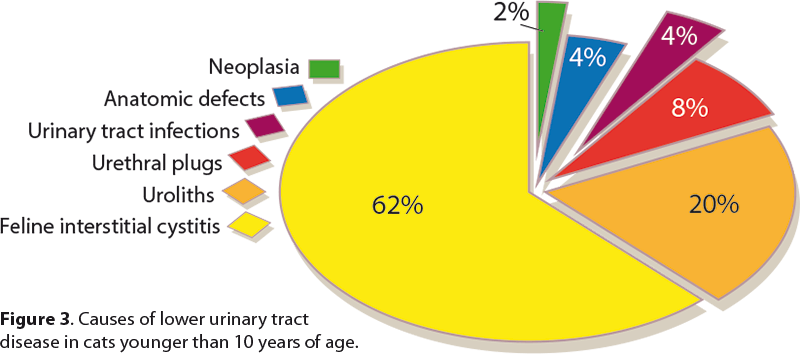
FLUTD includes cystic calculi, UTI, neoplasia, anatomic defects, behavioural problems and FIC. Urolithiasis comprises 15 per cent to 23 per cent of LUTD cases, 10 per cent to 21 per cent urethral plugs, anatomic defects up to 11 per cent, bacterial infections 1 per cent to 8 per cent and neoplasia up to 2 per cent (Sparkes, 2014; Figure 3).
In cats older than 10 years of age, bacterial infections account for 20 per cent to 50 per cent of cats with LUTD signs. Urine culture should be performed rather than assuming the urine is sterile (Bartges, 2002; Sparkes, 2014). Signs of LUTD in cats less than 10 years old without urethral obstruction may be caused by FIC in 55 per cent to 73 per cent of cases (Sparkes, 2014).
In humans, stress is associated with obesity, although it can be difficult to determine cause and effect between the two.
One-third to a half of adult cats are overweight or obese, and one study showed overweight and obese cats had a higher prevalence of LUTD, including FIC and urolithiasis, compared to normal weight cats (van de Maele et al, 2005).
Cats with FIC have a variable disease course, with signs usually resolving in five to seven days, then recurring. This waxing and waning is thought to be associated with events activating the central stress response system (Buffington, 2011). Many affected cats show signs from other organ systems; for example, the gastrointestinal tract, skin, lung, cardiovascular, central nervous, endocrine and immune systems. The disease aetiology likely has a neuropathic aspect, as well as local bladder wall disorders.
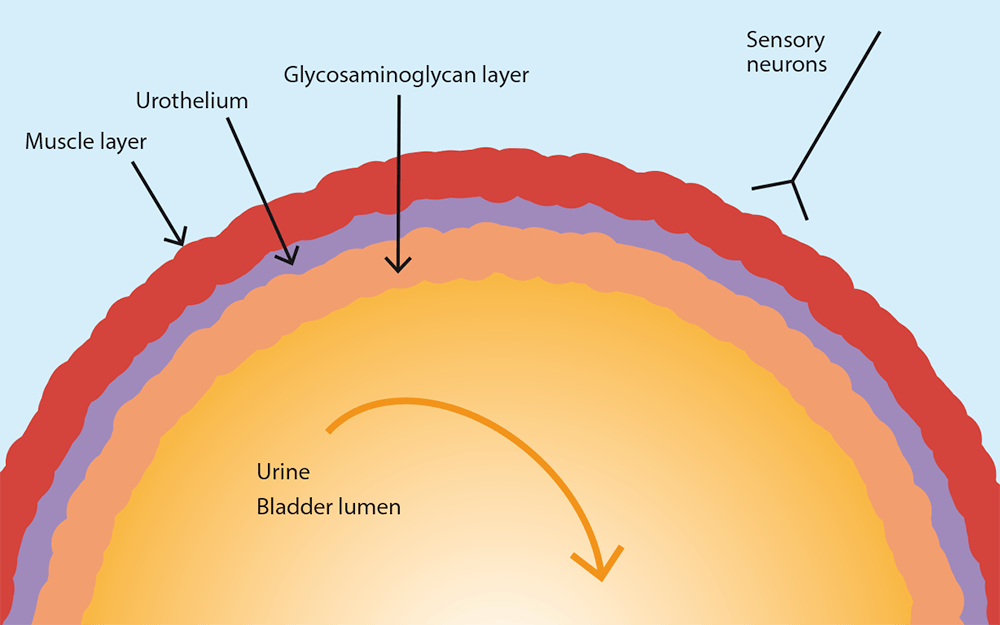
The bladder’s smooth and striated muscles, and the neurovascular supporting tissues, engage in complex neuroendocrine communication with the body and brain to coordinate urination. Bladder neural connections include sensory afferent; central; and somatic, sympathetic and parasympathetic efferent neurons interacting between the urothelium and the cerebral cortex (Buffington, 2011).
Cats with FIC show a denuded uroepithelium with increased permeability and a decreased total GAG layer in the bladder (Buffington, 2011). In a study of urine parameters, urine protein concentration was four times higher and urine protein to creatinine ratio five times higher in cats with FIC than in normal cats (Panboon et al, 2017). Increased serum concentrations of pro-inflammatory cytokines and chemokines are also present in FIC cats (Parys et al, 2018).
In addition to uroepithelial bladder abnormalities, urinary changes and altered serum cytokines in FIC, alterations in components of acetylcholine synthesis and release exist. Changes in the non-neuronal cholinergic system may contribute to alterations in cell-to-cell contacts, and communication with underlying cells that contributes to changes in sensory function and visceral (bladder) hyperalgesia.
Differences in sensory neuron anatomy and physiology are present in FIC cats, suggesting a more widespread abnormality of sensory neuron function. The acoustic startle response – a brainstem reflex motor response to a perceived threat from unexpected auditory stimuli – is increased in FIC cats.
Differences in sympathetic nervous system function identified in FIC cats include changes in the brain stem region associated with an important source of noradrenaline. This area is involved in brain functions – such as vigilance, arousal and analgesia – and mediates the visceral response to stress. Changes in brainstem help explain the waxing and waning of signs, and the aggravation of signs by environment stressors (Buffington, 2011).
Some cats with FIC have abnormalities in the hypothalamic-pituitary-adrenal axis, with decreased serum cortisol secretion and smaller adrenal glands compared with healthy cats. Therefore, some of these cats have an excessive sympathetic response to stress, with decreased cortisol response in addition to pathology within the bladder (Buffington, 2011).
Risk factors for FIC include being an indoor cat, young to middle age (four to seven years old), neutered and overweight. Other factors may include low activity, using a litter tray, eating a high proportion of dry food and living with more than one other cat – especially with conflict between the cats.
Compared to normal cats, those with FIC have been described as being more fearful, nervous, having less hunting behaviours, hiding when unknown visitors are in the house and drinking less water (Defauw et al, 2011). Episodes are often triggered by stress – for example, due to moving house, new cats in the house or neighbourhood, new people in the house or car rides to the clinic.
A case-control study focusing on indoor cats in South Korea showed increased FIC odds ratios (OR) for males compared to females (OR 2.34), cats not having vantage points to see out (OR 4.64), cats living in an apartment (compared to a house; OR 2.53), and, similar to previous studies, cats cohabiting with other cats compared to those living alone (OR 3.16).
Cats using non-clumping litter had 2.62 times the odds compared with those using clumping litter (Kim et al, 2017).
Generally, FIC cannot be cured, although it is often possible to decrease the frequency and severity of episodes. The initial treatment should include analgesics (such as buprenorphine) as these episodes are painful. Addressing stress management and diet are among the most important treatments.


A thorough history about feeding and management should be obtained. Resources can be found online (The Ohio State University, 2018). Multimodal environmental modification should be adopted to reduce stress, although only one or two changes should be made at a time.
Each cat should have its own food bowl, separate from the water bowl, and both should be away from the litter box. It is recommended the number of litter boxes equals the number of cats, plus one, although this can be challenging to place around a house (Figure 4). As non-clumping litter is identified as a risk factor, using a non-scented clumping litter and/or meticulous cleaning of the litter box may help (Figure 5).
The cat should be able to access all resources without competing with other cats. When cats are not compatible, microchip-operated cat flaps in internal doors can offer a means for separate, private access to feeding and litter boxes (Heath, 2016).
Environmental enrichment should also include resting and hiding places, provision of normal cat behaviours (such as scratching, hunting or play hunting), and a set routine with familiar people. The use of pheromone products can be helpful.
If possible, a wet diet should be fed and water intake increased. Water intake may be encouraged by daily fresh water and multiple full bowls placed away from food and litter boxes. Some cats prefer running water, such as a water fountain.
Many cats with FIC present with a urine specific gravity (USG) of around 1.050; decreasing the USG to less than 1.035 decreases the frequency of episodes (Gunn-Moore and Shenoy, 2004).
Two anti-anxiety ingredients used to treat FIC are L-tryptophan and milk protein hydrolysate (MPH). L-tryptophan is an essential amino acid and the precursor of brain serotonin. Increased serotonin is associated with increased sedation and decreased aggression, fearfulness, insomnia and pain sensitivity. In a double-blinded controlled cat study, added L-tryptophan decreased anxiety, stress-related behaviours and house soiling (Pereira, 2010).
MPHs – for example, alpha-casozepin – have a similar structure to gamma aminobutyric acid – an inhibitory neurotransmitter that decreases anxiety and stress-related disorders. MPH given to cats orally decreases fearful behaviours and increases contact with people (Beata, 2007).
A study showed beneficial effects of an alpha-casozepine and L-tryptophan-supplemented diet on fear and anxiety in cats placed in an unfamiliar location, although fear in the presence of an unfamiliar person did not decrease (Landsburg et al, 2017). A urinary food supplemented with MPH and L-tryptophan fed for eight weeks to 18 cats with FIC improved FLUTD signs, and emotional and quality of life scores (Meyer and Bečvářová, 2016).
In summary, feeding a wet food and/or a food or ingredient to decrease stress has good evidence for decreasing the signs of FIC. Antioxidant therapy also appears to help and glucosamine may have some benefit.
Overweight cats are more at risk and, although weight loss has not been studied as a therapy, it is recommended due to the risk of comorbidities of obesity.