1 Feb 2022
Samuel Mauchlen BVM&S, MRCVS and Chris Dixon BVSc, CertVOphthal, MRCVS conclude their article by focusing on the appearance of pathological conditions.
Ocular ultrasonography is a safe and valuable technique for assessing the ocular structures.
However, given that probe placement involves direct or indirect pressure on the corneal surface, this imaging modality may be contraindicated in cases where severe globe or corneal trauma has occurred, or where ocular surgery has recently been performed.
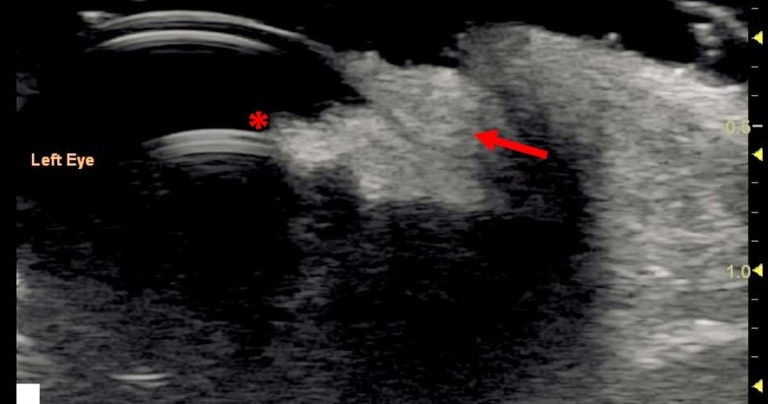
When using a medium to high‑frequency transducer (12MHz to 20MHz or higher), the three-layered nature of the cornea can be visualised.
Generally, any disruption to the anterior layer would be directly visible with an ophthalmic examination (for example, corneal ulceration or sequestrum), but high‑resolution ultrasound scanning can allow for the detection of conditions that may be “hidden” due to opacification of the superficial layers (Figure 1).
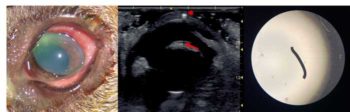
Corneal thickness can be accurately measured with an ultrasonographic pachymeter, but portable “pen” devices are expensive and only normally found in ophthalmic referral centres (Figure 2).
Alternatively, using a transducer higher than 12MHz may allow for measurement during B-mode scanning, provided the depth is reduced as much as possible and frequency increased to maximise resolution of the anterior structures. Flattening of the cornea will occur if excessive pressure is applied (Figure 3). This can be avoided with liberal application of a sterile acoustic coupling gel.
The normal canine corneal thickness is approximately 0.57mm1 and this can increase in conditions that result in corneal oedema due to hydration of the corneal stroma.
Ocular ultrasonography should not be performed if evidence exists of significant corneal ulceration. Direct pressure from the transducer could potentially lead to corneal rupture in cases with a deep stromal ulcer or descemetocele.
Pathology affecting the anterior chamber can be visualised as echogenic lesions within the normally anechoic chamber (Figure 4). Echogenic material can represent haemorrhage and cellular debris (for example, hyphaema or hypopyon) and can be mobile, changing position with ocular motion.
Iridociliary cysts can be identified using ultrasonography (Figure 5). These form from the neuroepithelium of the uveal tract in the posterior chamber and can “float” into the anterior chamber. The cysts are roughly spherical and on ultrasound appear as thin echogenic walls with an anechoic centre. Golden retrievers, Labrador retrievers, great Danes and Boston terriers are predisposed, although other breeds can be affected.
Small individual iridociliary cysts are usually considered to be an incidental finding, although larger cysts can obscure vision, and an accumulation of cysts has been associated with uveitis and glaucoma2,3.
Ultrasound can be used to assess the lens and can be particularly useful in cases of cataract formation.
Caused by degenerative changes in the solubility of lens proteins, cataracts can be classified based on their location within the lens and the volume of the lens that the changes occupy. Changes in lens thickness in cataract cases have been described4.
On ultrasound, cataracts appear as echogenic areas within the normally anechoic lens (Figure 6).
Ocular trauma, glaucoma, chronic uveitis, neoplasia, and conditions that affect lens volume or stiffness can lead to stress and eventual rupture of the zonule fibres that support the lens. This loss of support can cause subluxation or full luxation of the lens.
Subluxation may allow the lens to move or wobble (phacodonesis). The position of the lens may appear asymmetrical on ultrasound examination, although it will remain immediately posterior to the iris and anterior to the vitreous body.
Lens luxation will allow the lens to move completely out of its normal position. If it moves into the anterior chamber, it can be visualised immediately posterior to the cornea (Figure 7). If the lens moves posteriorly, it will be visible within the vitreous body (posterior segment; Figure 8).
A fully luxated lens will be metabolically stressed and often can appear cataractous, exhibiting internal changes such as those described previously.
The vitreous chamber normally appears anechoic. Echogenic opacities within the chamber can be caused by a number of different conditions.
Asteroid hyalosis is caused by calcium lipid complexes that are suspended throughout the vitreous, and are a common cause of vitreal opacities in older patients, brachycephalic breeds and Italian greyhounds (Figure 9).
Other forms of vitreal degeneration can be visualised ultrasonographically5 and are commonly seen in dogs with cataract formation.
Vitreal opacities can also be seen in cases of inflammation and infection (Figure 10).
Haemorrhage within the vitreous chamber can cause the formation of echogenic opacities or an echogenic mass. Causes of haemorrhage include:
Organised clots within the chambers of the eye can be difficult to distinguish from neoplastic lesions7. In these cases, Doppler ultrasonography can be used to distinguish between the active flow in a vascularised neoplastic lesion and the lack of flow in an organised clot.
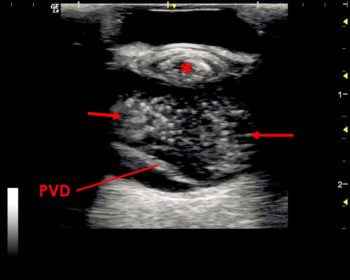
Retinal detachment can have a characteristic appearance and is commonly diagnosed using ultrasonography. The retina is not normally distinguishable from the other posterior layers of the globe wall.
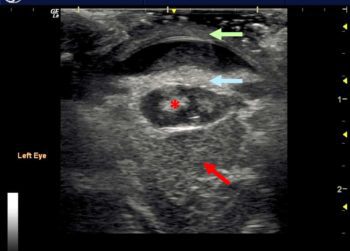
Detachment appears as a thin echogenic interface within the vitreous chamber, with underlying anechoic space separating it from the combined choroid and sclera.
The retina usually remains attached at the optic disc posteriorly and the ora serrata near the ciliary body anteriorly. Complete detachment will lead to a “V” or “Y”‑shaped appearance within the vitreous chamber (Figure 13).
Retinal detachment can occur secondary to severe inflammation of the choroid. Although this layer is difficult to evaluate, in cases of severe inflammation the choroid becomes oedematous. This can be visualised as thickened hypoechoic layer between the sclera and retina (Figure 14).
Within the area denoted by the red circle, the normally combined appearance of the posterior tissue layers has been lost.
From anterior to posterior, the layers seen are the echogenic interface of the detached retina, an anechoic area of subretinal oedema, a thickened choroid with a band of hypoechoic choroidal oedema, and the scleral layer.
Intraocular neoplasia can be focal or diffuse and affect different parts of the eye (Figure 15).
Additionally to visible masses and structural changes to the ocular anatomy, neoplasia can cause glaucoma, haemorrhage and uveitis.
Ultrasound is extremely valuable in evaluating neoplastic pathology as it can help to define the extent of the pathology, especially when normal structures are obscured.
Choroidal melanocytic neoplasia is the most common ocular neoplasia seen in dogs and cats8,9. Ciliary body adenomas/adenocarcinomas are the second most common in dogs10.
Post-traumatic fibrosarcomas are seen in cats. Less commonly, haemangiosarcomas, lymphoma, medulloepithelioma, astrocytomas and meningiomas are seen.
The ultrasonographic appearance of foreign bodies will vary depending on their composition.
Ultrasound can be used to establish their presence, but also to evaluate the position of foreign objects prior to removal.
Other imaging modalities – such as radiography, CT and MRI – can also be useful in evaluating orbital foreign bodies.
Ultrasound examination can be very rewarding and is a very useful first‑line technique for evaluating the retrobulbar structures11 following a complete ophthalmic examination.
CT and MRI can also be valuable for evaluating the retrobulbar anatomy, although availability and expense may limit their use. Additionally, radiography should be considered to assess bony involvement in pathology.
Neoplasia can be primary – from the structures within and around the retrobulbar space – or secondary with metastatic spread.
The ultrasonographic appearance of neoplasms can vary in echogenicity and masses can cause deformation of the posterior globe.
Inflammation of the retrobulbar structures can vary in appearance (Figure 16). Diffuse cellulitis can result in the retrobulbar fat appearing more hyperechoic.
The change in tissue echogenicity can be subtle, and it is always useful to compare the affected and normal eye when assessing the retrobulbar structures.
Abscesses and cystic lesions can also be seen within the retrobulbar space. These may have an echogenic rim with hypoechoic or flocculent content.
The zygomatic salivary gland is located ventral to the retrobulbar tissue, and cases of sialocele or sialoadenitis can cause enlargement of the gland and protrusion into the retrobulbar space – this can also appear as a cavitary lesion.
In cases of retrobulbar disease, ultrasound can be used to guide fine needle aspiration to avoid damaging the neighbouring structures, such as the globe, optic nerve and blood vessels. This technique can also be used in the drainage of cavitary lesions.
Ocular ultrasonography is an extremely important and versatile diagnostic modality that can be performed safely in the majority of patients.
Ultrasonography can reveal information that would otherwise have remained unknown with direct visualisation of the eye and can enhance the clinician’s ability to reach an accurate diagnosis.