23 Jul 2024
Nick Bacon shares some of the latest knowledge on cancer topics, including managing it early on.

Oncology is a rapidly evolving discipline, and the need and desire to stay current from the clinician’s perspective is often only matched by the search engine capacity of the pet-owning public.
A poll by the Oncology Working Group of the WSAVA of almost 2,000 veterinary health care workers identified that they rated their own knowledge of oncology as just 5 out of 10, yet ranked the actual importance of oncology cases for their practice at 7 out of 10, with minimal variation (6.3 to 7.7) between languages and global regions.
A huge demand remains for veterinary oncology education generally, and this article will hopefully share some of the latest knowledge in early diagnosis, staging and treatment.
Blood, urine or other bodily fluids may help diagnose the presence of cancer (for example, similar to the prostate-specific antigen test in men for prostate cancer).
An accurate, minimally invasive, sensitive (ability to designate an individual with disease as positive; that is, few false negatives) and specific (ability to designate an individual who does not have a disease as negative; that is, few false positives) liquid biopsy is considered by many as the holy grail for personalised cancer care in humans and animals. As such, it is a highly attractive area of research, development and investment.
For decades, we have all been asked by owners, “Isn’t there a blood test for cancer?”, and so it is clear the demand from patients and clinicians already exists. The vision is to detect cancer before symptoms have arisen, or demonstrate cancer is truly in remission, allowing for better decision making, more appropriate and timely deployment of both clinical and financial resources, and likely improved outcomes and patient welfare. Slowly and steadily, the profession is being offered more options, with several companies working in this space. The true role of liquid biopsies is as yet unclear, but all applications are likely to be based around three key questions.
● Screening
● Impact. Following a treatment, such as surgery or chemotherapy, has all the cancer gone and is the patient now in remission?
● Monitoring. Have we remained in remission or does evidence show the cancer is returning?
The first point to make is that it is highly unlikely that one single test will be the perfect choice in every clinical scenario. It is also unlikely that liquid biopsies will provide stand-alone “proof”, but will be used in conjunction with more conventional diagnostics. As veterinary surgeons, we must remain open-minded and patient as this field grows and the results of clinical research mature. We should not rapidly and entirely dismiss one type of test simply due to poorer than desired results in one particular disease setting.
Blood liquid biopsies, either available, in development or currently unavailable, include:
● NuQ Vet (www.veterinary.volition.com). This is very much targeted as a screening test for healthy, asymptomatic middle to older patients, who have disease, but do not yet have symptoms. It may also be appropriate for younger dogs in breeds with known increased risk of cancer. The technology uses antibodies to detect nucleosomes; small DNA wrapped packages (histones) released into the blood when cancer is present.
Requiring only 0.5ml plasma, the test can reportedly detect haemangiosarcoma and lymphoma prior to the onset of symptoms, with mast cell tumours, melanoma and histiocytic sarcomas also being detected in some patients.
In a study of 662 dogs, NuQ Vet Cancer Test could detect 50% of all cancers researched with a 97% specificity, and 76% of systemic cancers, such as lymphoma and haemangiosarcoma (Wilson-Robles et al, 2022). Research is continuing, as increased nucleosomes can also be seen with systemic inflammation, sepsis, trauma and immune-mediated disease.
● K9-LiquiDX (CanCan Diagnostics; www.cancandiagnostics.com). This is a DNA-based test spun out from research at The University of Edinburgh. The test captures circulating cell-free DNA in the blood (DNA fragments released when cells die), and a small fraction of this might come from tumour cells. The test is targeted more towards supporting treatment of cancer in dogs as a monitoring tool or to support difficult diagnoses; that is, measuring plasma tumour DNA at the point of diagnosis to set a baseline, and then comparing to future samples to monitor the impact of treatment, or the progress of disease. It is hoped that in the future it may be useful for screening and early detection.
● Oxford BioDynamics (www.oxfordbiodynamics.com). A peripheral blood sample is used to detect and diagnose six common canine cancers: melanoma, osteosarcoma, haemangiosarcoma, histiocytic sarcoma and lymphoma (B and T cell). The technology detects 3D genomic markers to identify abnormalities indicative of a particular cancer and, in humans, can even predict likelihood of a response to particular immunotherapy treatments. Early results are encouraging, but front-line veterinary testing in a clinical environment is underway, with results to follow.
● OncoK9 (Pet Dx – currently unavailable). This test showed much early promise, by performing next-generation sequencing of cell-free DNA to detect multiple genomic alterations in dogs with cancer. Sensitivity was 61.5% and specificity was 97.5% – that is, if negative, the dog likely did not have cancer (Kruglyak et al, 2021; Flory et al, 2022). No data exists regarding when this test will be back on the market.
In addition to peripheral blood, urine can be used as a liquid biopsy, with all the added benefit of ease of sampling – especially in the home environment. Urine liquid biopsies include:
● CADET (Antech; www.antechdiagnostics.com). This test uses free-catch urine to support a suspicion of urothelial carcinoma in dogs, either due to suggestive clinical signs or lower urinary diagnostics such as identifying a bladder or prostatic mass. The test could also be used to screen high-risk breeds, such as the Scottish terrier. It measures a mutation in the BRAF gene of cancer cells shed in the urine of dogs with bladder or urethral carcinomas. Reporting a sensitivity of 85% and specificity of more than 99%, it can be helpful in the diagnosis and management of these challenging cases (Mochizuki et al, 2015).
● Oncotect (oncotect.co). This uses the chemotactic attraction of a nematode towards urine from cancer-bearing dogs. Volatile organic compounds are by-products of cancer cell metabolism, and the Caenorhabditis elegans has been shown to be strongly attracted to urine samples from dogs with lymphoma, melanoma, haemangiosarcoma and mast cell tumours. A positive nematode response identifies a clear cancer risk for that sample, with an 85% sensitivity and 90% specificity. The specific type of cancer is not identifiable, however (Namgong et al, 2022).
The test is marketed as a screening tool for healthy animals seven years or older, or younger animals of at-risk breeds. Work is continuing on the impact of urinary inflammation and infection on test results so, similar to all liquid biopsies, results should never be used in isolation.
“In addition to peripheral blood, urine can be used as a liquid biopsy, with all the added benefit of ease of sampling.”
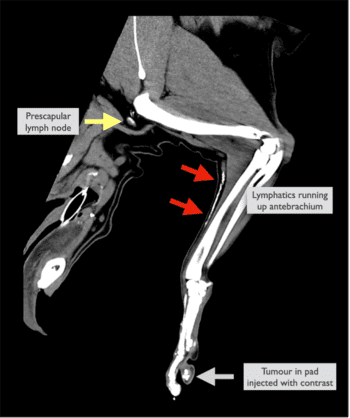
The pattern of cancer metastasis varies between tumour types, with epithelial and round cell tumours tending to initially prioritise lymphatic spread, and sarcomas metastasising preferentially via the blood to the lungs. This means that for a huge number of commonly encountered tumours in veterinary practice (such as mast cell tumours, melanoma, mammary carcinoma, squamous cell carcinoma or anal sac tumours), accurate identification of the lymph node draining the tumour, the so-called sentinel lymph node (SLN), is vital.
It is not always the geographically nearest, and failure in this respect results in the wrong lymph node being sampled, resulting in treatment delay, incorrect planning and, at worst, a poorer prognosis if metastatic disease inside a node is left behind. Regional lymph node assessment is well recognised in human oncology to maximise patient outcome, but in veterinary medicine, we have historically relied on palpation and ultrasound. This can result in up to 60% of cases having malignant cells potentially missed in their sentinel lymph node sampling, and the diagnosis of metastatic lymph nodes may be incorrect in more than 85% of cases if lymph node assessment is performed by palpation alone (Brissot and Edery, 2016). The past decade in veterinary medicine has witnessed a growing interest in sentinel lymph node mapping techniques:
● Radiographic lymphangiography (pre-op)
● Contrast-enhanced ultrasound (pre-op)
● CT lymphangiography (pre-op)
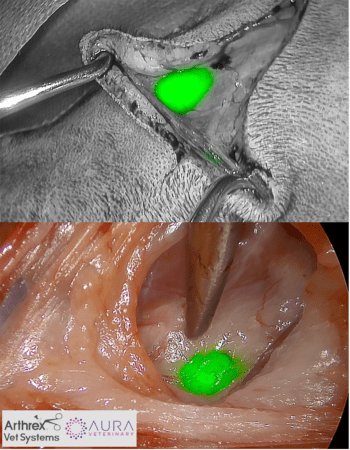
● Colorimetric mapping (real-time in surgery)
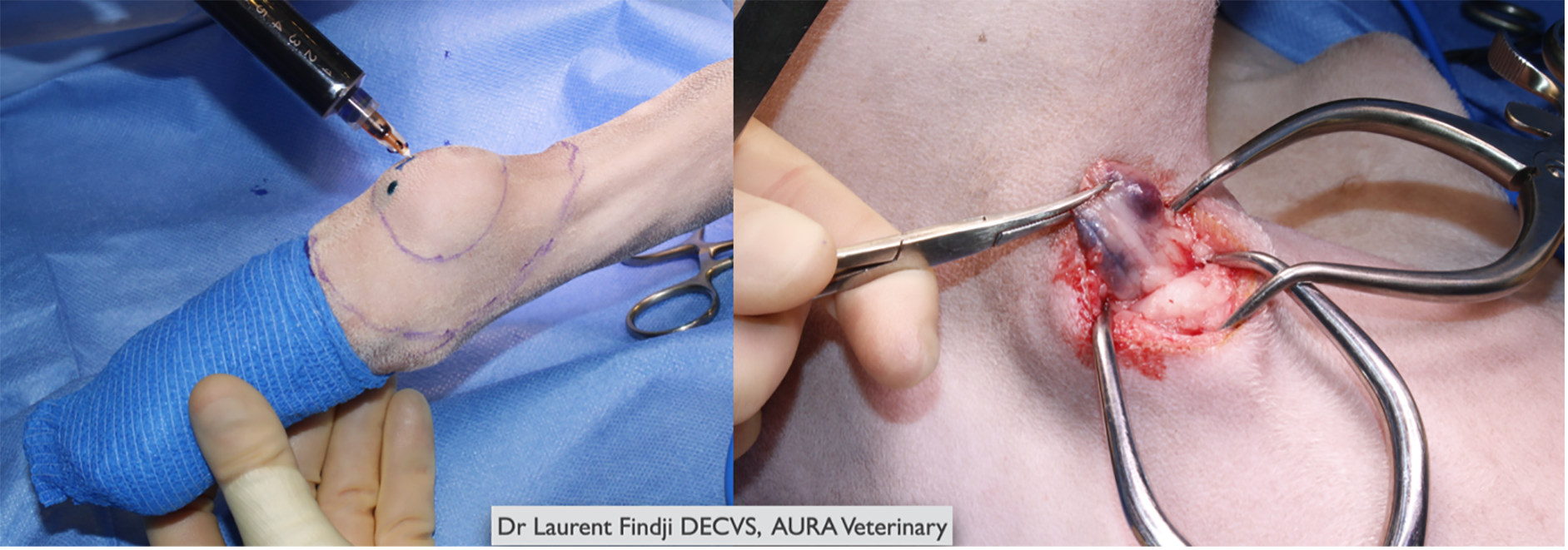
● Near infrared fluorescence (NIRF; real-time in surgery)
Thermal destruction of tumour cells can be achieved by inserting a narrow needle into the centre of a mass, often under ultrasound or CT guidance, and then the tip being energised to create a thermal ablative sphere.
Repositioning and reheating the needle allows for large irregular shapes to be ablated. Microwave ablation (MWA) is frequently used in humans, often for lung, mammary, liver and renal tumours, and has similar applications in veterinary medicine. The needle is often inserted under image-guidance percutaneously, although it can be directed into a tumour directly; for example, at open surgery. In studies in dogs with liver tumours, no complications were recorded and overall response was encouraging. MWA has also been described for retroperitoneal neoplasia in dogs (Culp et al, 2021) and the lung tumours at the author’s hospital.
“Prevention is better than a cure”, attributed to the philosopher Erasmus, hardly seems insightful in modern medicine, but it could never be truer than in the complexity of contemporary cancer care. The company Calviri studied neoantigens (irregular peptides from mutations in tumour DNA) and identified some of these unique protein fragments that are shared across multiple canine cancer types.
The company manufactured a vaccine containing 31 of these shared neoantigens, and ran a clinical trial to study their efficacy, called the Vaccine Against Canine Cancer Study.
This five-year study was the largest of its kind in veterinary oncology and was successfully concluded on 4 May 2024. It involved 800 dogs at 3 centres and was double-blinded, with dogs receiving a vaccine and yearly booster, or a placebo (Burton et al, 2024). The safety and efficacy results were very encouraging, and now the study is in a monitoring phase to analyse the impact of the vaccine on cancer incidence, and results will be published in due course. Read more at tinyurl.com/2ncb24wx
Oncept melanoma vaccine (formerly Merial, now through Boehringer Ingelheim in the UK) was the first widely used cancer vaccine in veterinary medicine for melanoma in dogs. The human melanocyte protein tyrosinase is very similar to the same enzyme in dogs and is part of normal pigment production.
The Oncept vaccine contains DNA from tyrosinase, which causes the dog to mount an immune response against human/canine tyrosinase; therefore, targeting melanoma cancer cells, and often normal pigment cells.
Approval by the United States Department of Agriculture confers safety rather than necessarily efficacy, and it is approved for treatment of advanced (metastatic) oral melanoma, and is off-label for other locations and other species. Many oncologists have experienced mixed results in their patients, with both astounding “successes” and disappointing “failures” seen.
Typically, however, like all vaccines, several months are needed before a full immune response can be mounted in a patient, and so the vaccines are best given in a microscopic setting; that is, once all detectable cancer has been removed. Treating large bulky tumours with a vaccine is rarely beneficial. Vaccines are given every two weeks for four doses, then every six months. Pellin (2022) recently published a valuable review of the Oncept literature.
APAVAC (www.vetlig.com). With the aid of a single-use veterinary medical device, an autologous vaccine can be made in your clinic under sterile conditions, using tumour tissue taken directly from the patient to make a patient-specific vaccine. The author’s clinic uses APAVAC for lymphoma (Marconato et al, 2019), osteosarcoma and melanoma when more conventional treatments have been declined or failed. A 0.5cm3 cube of tumour should be collected in a dry, sterile, plain universal container and stored in a -20°C fridge. The vaccine is given subcutaneously once a week for four weeks, then once a month for four months.