5 Mar 2018
Bob Partridge discusses the pathogenesis of periodontal disease in dogs and cats, focusing on biofilms and plaque.
This article discusses biofilms and how their unique capabilities provide a protective and adaptive environment for the contained bacteria. Plaque, the specialised oral biofilm, is discussed in detail.
The oral bacterial flora biome has been the subject of intensive research, with new equipment providing a fresh outlook. The previous assumption cat and dog biome would mimic that found in humans has been disproven by workers at the Waltham Centre for Pet Nutrition. Dramatic differences to previous perceived knowledge are outlined.
The development of calculus from plaque mineralisation is described and illustrated with a video. Periodontal disease results from the interaction of the plaque biofilm and the host inflammatory response. The processes are described, together with potential systemic and environmental influences.
In part two, treatment and prevention will be covered.
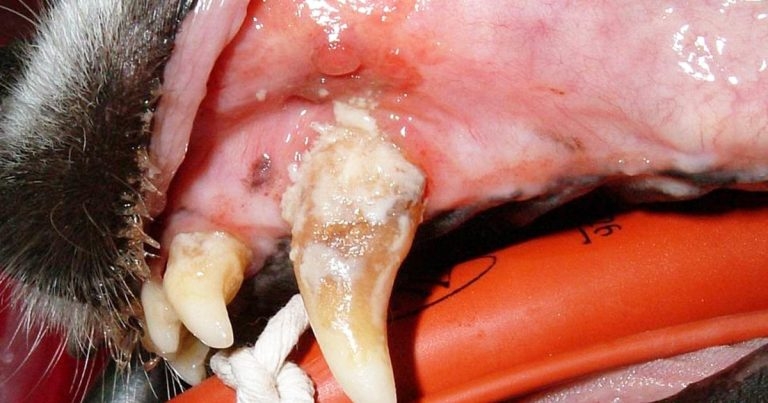
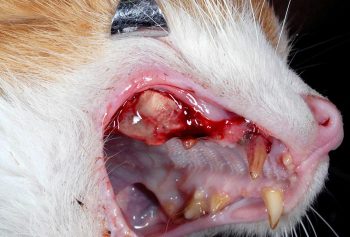
Periodontal disease is the most common dental disease affecting cats and dogs. An understanding of the origins of periodontal disease allows logical and effective treatment to be implemented.
Canine dental disease can be categorised in a number of different forms:
While each of these is fascinating in itself, arguably the most important is periodontal disease. An understanding of the origins of periodontal disease allows clinicians to take a logical and effective approach to treatment, not simply relying on antibiotics.
It has become widely accepted periodontal disease results from interactions between the complex biofilm plaque with its mixed bacterial content and the host’s own inflammatory response. Most assumptions have been based on human research findings, but we know – thanks to investigative research at the Waltham Centre for Pet Nutrition – human plaque biome is very different from that found in cats and dogs in health and disease.
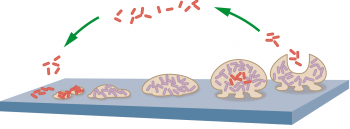
Biofilms are almost ubiquitous, from oil pipelines in Alaska to indwelling catheters in our patients. They may be simplistically considered as a sugar and protein slime matrix within which bacteria are embedded. The slime (extracellular polymeric substance; EPS) is synthesised and secreted by the microbes.
Bacterial DNA from both living and dead microbes also forms an important part of the EPS. The bacterial community (or biome) contained within the matrix is complex and diverse. The composition and ratio of its constituents can change with both the age of the biofilm and circumstances.
Biofilms grow very rapidly. Planktonic (or free-living) bacteria attach to surfaces within a few minutes, then form strongly attached microcolonies within two to four hours. Initial EPS formation starts within 6 to 12 hours, creating a mature biofilm, which is highly resistant to biocides and sheds fresh planktonic bacteria and portions of biofilm to create fresh communities within two to four days.

The EPS attaches the community to either living or inert surfaces. However, the EPS has a number of other important functions. It provides a protective buffering environment; physically large molecules, such as antibodies, may not be able to penetrate the EPS to access the microbes.
Even smaller molecules, such as antibiotics, may be prevented from diffusing through the matrix – doses that would kill free-living bacteria would often have no effect on those within a biofilm. The EPS allows sharing of resources – an antibiotic-resistant bacterium may secrete a protective enzyme, which may benefit neighbouring bacteria within the EPS.
The EPS may also facilitate plasmid transfer of resistance genes between species. Similarly, chemical messengers can allow communication within a species colony and between colonies of different bacterial species. The phenomena of quorum sensing is a change in gene expression, such as a number of bacteria coordinating a phenotypic change that can result in “swarming” or a rapid spread of the biofilm across a surface.
Biofilms almost have to be regarded as entities in themselves, rather than simply groups of many different bacterial species.
Plaque is the specialised biofilm within the oral cavity. Its formation is assisted by the presence of the pellicle – a thin layer of glycoproteins that coats the entire oral cavity. It derives from saliva and gingival crevicular fluid (GCF; the transudate and inflammatory exudate produced in the gingival sulcus surrounding the teeth).
Primary colonisation occurs when planktonic bacteria attach on to the pellicle – secondary colonisation then follows. The bacteria in this second wave lack the ability to attach to the pellicle, but are able to attach to the bacteria that form the primary colonisers – a classic example of the cooperative nature of biofilms. EPS becomes more abundant, creating the protective biofilm environment. Fresh planktonic bacteria and portions of the biofilm itself are shed by the maturing biofilm to colonise new areas.
After thorough mechanical disruption of an attached mature biofilm (such as by efficient tooth brushing), the pellicle will reform in nanoseconds and a mature biofilm will be re-established within 24 hours.

Our knowledge of the bacteria that constitute plaque is limited by the available technologies. Initially, aerobic, anaerobic and microaerophilic culture methods have been developed. However, it is accepted only 3% of the bacteria present are able to be cultivated.
The development of 16S ribosomal RNA cloning allowed a greater understanding of the phylogeny and taxonomy of the complex microbiome. It was the drive to sequence the human genome that created next generation sequencing technology, which has allowed far greater identification of the microbes present. The world-leading research carried out at Waltham is challenging the accepted idea that the components of plaque in health and disease in veterinary patients are similar to those of humans.
The accepted human model is the primary colonisers of plaque are aerobic, Gram-positive cocci and short rods; typically, Streptococcus and Actinomyces species. These bind specifically on to the pellicle. The secondary colonisers cannot adhere directly to the pellicle, but attach to receptor molecules on the primary colonisers. The secondary colonisers are typically anaerobic, Gram-negative rods and cocci, with filamentous spirochaetes and flagellated rods present (Table 1).
Porphyromonas species, such as Porphyromonas gingivalis and Porphyromonas intermedia, are considered pathogenic.
In dogs, unlike humans, streptococci do not play a role in the primary colonisation. Instead it is predominantly the Gram-negative diplococcal species of Neisseria, along with Corynebacterium species and Stenotrophomonas species (Table 2).
In healthy canine mouths, Gram-negative bacteria predominate. Porphyromonas, Moraxella and Bergeyella species predominate in healthy plaque – Streptococcus species are much rarer than in human samples.
As mild periodontitis develops, Gram-positive anaerobic bacteria predominate – with Peptostreptococcus, Actinomyces and Peptostreptococcaceae becoming abundant. In general terms, the total variety of bacterial species are present in both health and disease; however, the relative proportions change as disease progresses. Loosely, the proportion of “healthy” bacterial species decreases with the onset of disease states.
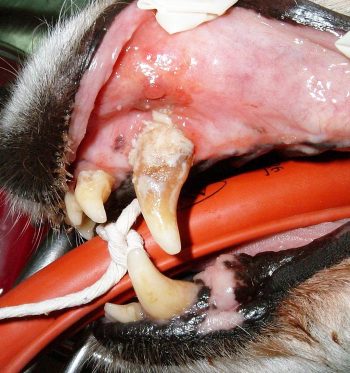
Mineralisation can start as soon as four hours after plaque formation, or certainly within a few days. The result is the formation of large, irregularly surfaced structures with 70% to 90% inorganic components (mostly hydroxyapatite) both supra-gingivally and sub-gingivally.
Supra-gingival calculus’ organic component stems mostly from saliva; its attachment is to the pellicle covering the tooth enamel. This is relatively weak and explains why supra-gingival calculus can often be easily “cracked off”. This has contributed to the development of “non-anaesthetic” dentistry, the purely cosmetic benefit of which has been condemned by the RCVS. Sub-gingival calculus derives much of its organic content from gingival crevicular fluid, often resulting in a darker-coloured material.
In addition, sub-gingival calculus is more intimately bound into tooth cementum, sometimes even entering the dentinal tubules. Sub-gingival calculus is, therefore, more difficult to remove, often requiring the removal of some cementum.
While the physical bulk and rough surface of the calculus can cause local inflammation, the major effect is of increasing the available surface area for more plaque formation. Where the calculus accumulates in the periodontal pocket it can assist in weakening the gingival attachment by physically pushing the gingiva away from the tooth.
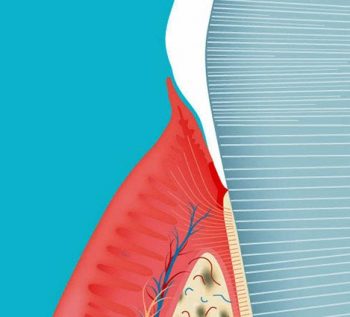
This is highly complex and involves many interactions between plaque and the host tissue response. Plaque bacteria produce metabolites, which stimulate the junctional epithelium (the boundary between the tooth and the gingiva at the base of the gingival sulcus) to synthesise inflammatory mediators.
Nerve endings produce neuropeptides and histamine, leading to the release of interleukin 8 and the attraction of polymorphonuclear leukocytes (PMN). Local blood vessels dilate, slowing the blood flow, and allow the streaming of PMNs towards the blood vessel walls. They attach to selectin molecules on the endothelium, the wall becomes more permeable and migration of PMNs occurs with subsequent chemotaxis through the connective tissues towards the plaque in the sulcus.
An increased production of matrix metalloproteinases occurs, leading to degradation of the connective tissues and periodontal fibre network. Locally stimulated macrophages, with complement activated by plasma cells, with other inflammatory mediators leads to destruction of the organic bone matrix. This, together with inhibition of osteoblasts and proliferation of osteoclasts, leads to periodontal alveolar bone destruction.
In addition to the local inflammatory processes, other risk factors come into play. In humans, certain genetic defects (such as Down’s syndrome) are associated with increased incidence and severity of periodontal disease. Certain breeds, such as Maltese terriers, are known to have a predilection for periodontal disease, and, anecdotally, there also seems to be some familial susceptibility.
More research is required to identify if any genetic markers can be identified. Diabetic patients have a tendency to overproduce prostaglandin E2 and have defective function of PMNs and decreased antibody production. Renal disease can lead to impaired metabolism of gingival tissues, while pregnancy is also linked to some forms of periodontal disease. Some drugs produce changes in periodontal tissues. Immunosuppressive illnesses are also likely to predispose patients.
The biggest environmental factor relating to periodontal disease in humans is smoking. Research shows pets are more affected by passive smoking than humans living with a smoker. It is certainly possible smoking’s deleterious effects on PMN function, antibody production, bone and connective tissue metabolism may be a factor in veterinary dentistry.
Xerostomia, or dry mouth, can result from specific diseases of the salivary glands (such as Sjögren’s syndrome), systemic diseases, such as diabetes or hepatitis; or from the prolonged use of certain antihistamines, antihypertensives or antidepressant medications.
In our patients, prolonged mouth breathing can also have the effect of drying the mouth and decreasing the protective effect of saliva. Trauma, either from the irritation of hair trapped periodontally by excessive grooming, or to gingival tissues or partial tooth avulsions, will encourage periodontal disease.
Whether “upbringing and socioeconomic” factors, which are an important factor in humans, correlate with any effect on rescue animals is unknown.
The previous assumption canine and feline periodontal bacterial flora would mimic that of humans has been shown to be false. The key goal of treatment is to remove plaque and structures, such as calculus, that encourage its retention and development.
Success can only be achieved if suitable preventive measures are established. These may include surgical changes to periodontal anatomy, daily brushing, a change to preventive diets, use of special chews or the use of validated drinking water additives.
Innovative approaches, such as the use of a sealant applied to the gingival sulcus, offer an exciting alternative treatment. These will be detailed in part two.
The author would like to thank Ian Davis, Corrin Wallis and Lucy Holcombe of the Waltham Centre for Pet Nutrition for their time and generously sharing their research findings.