24 Sept 2018
Mathilde Granger discusses management, care and rehabilitation considerations when dealing with traumatic brain injuries.

Figure 1. Increasing sensory input by wearing feet wraps, for instance, may help manage stress and hypertonicity.
In September 2017, the British Veterinary Rehabilitation and Sports Medicine Association (BVRSMA) held its annual conference on traumatic brain injury (TBI) and rehabilitation.
A panel of specialists – vets Nicolas Granger, Gwen Covey-Crump and Lowri Davies and RVN Holly Mitchell – talked about the neurological, anaesthetic, pain management, nursing and rehabilitation aspects of TBI.
TBI in small animals occurs in up to 25% of blunt trauma. While severe head trauma is associated with a high mortality rate, less traumatic injuries are often overlooked, but can have particularly disabling consequences – hence why every patient should have a detailed neurological examination, and be given an appropriate treatment and a bespoke rehabilitation programme.
The main causes of head trauma are road traffic collisions, falls, collision with an object or other animal and bite wounds. The subsequent brain damage is the sum of the primary trauma to the brain, and secondary lesions due to inflammation, oedema and ischaemia.
Primary trauma may be the consequence of blunt trauma to the skull (skull fracture), perforation of the skull (bite), collision of the brain tissue against the skull and/or shearing of the brain tissue due to rotational or translational forces.
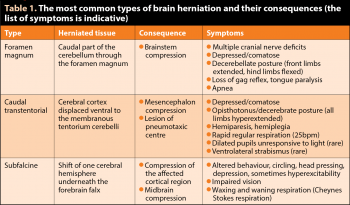
If secondary lesions lead to brain swelling exceeding the volume of the skull, the brain tissue is further damaged by compression and the parenchyma can herniate (Table 1). The symptoms depend on the severity of the trauma and the affected parts of the brain.
Firstly, head trauma patients are often polytraumatised, so should be thoroughly examined for concurrent conditions. Although the first neurological examination is an important baseline, this may be greatly worsened by the shock status of the patient. This should be considered cautiously before giving the owners a poor prognosis.
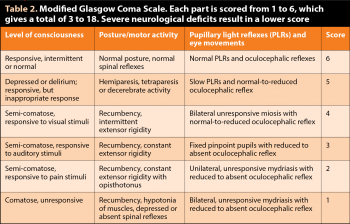
The modified Glasgow Coma Scale (Table 2) is easy to implement and useful to objectively monitor improvement or worsening in the early hours post-trauma (for example, repeated up to every 30 minutes to 60 minutes initially). But the prognostic value of one isolated result is debatable. The primary concern, in the initial stage post-trauma, is to maintain adequate brain perfusion to support normal oxygenation and limit secondary lesions. This implies maintaining a normal blood pressure (BP; mean arterial BP 80mmHg to 120mmHg) and supporting a normal ventilation.
Hypercapnia and hypoxia will lead to vasodilation – potentially increasing intracranial pressure – and should be avoided. A mild hyperventilation to maintain a mild hypocapnia (30mmHg) is advised. Respiration rate, arterial BP, pulse oximetry and, where possible, capnography, should be regularly monitored.
The second focus is analgesia. TBI animals can struggle to express pain, which may lead to poor patient cooperation and secondary hypertension. Regardless of severity, 50% of humans with TBI have post-traumatic headaches.
Greater benefits to treat pain exist than perceived risks, such as respiratory depression. Methadone and buprenorphine are safe to use, with minimal effect on cardiac output, respiration and BP, and have a mildly antitussive effect. Methadone can also be titrated to effect. In case of increased intracranial pressure, the mean arterial pressure will increase to maintain a normal cerebral blood flow. This is perceived by the aortic arch baroreceptors causing a reflex bradycardia – the Cushing reflex.
In this instance, reducing the mean arterial pressure would be detrimental, as it would further reduce the cerebral blood flow. Instead, every effort should be made to reduce intracranial pressure. Mannitol is the treatment of choice – 0.5g/kg to 1g/kg over 15 minutes – associated with moderate hyperventilation.
These patients should always be stabilised before considering imaging and surgical treatment if indicated. If anaesthesia is required, anaesthetic agents that provide a rapid controlled induction, suppression of the cough reflex, and minimal cardiac and respiratory depression should be used.
Finally, seizures may occur and should be swiftly treated. In practice, seizures of more than five minutes without recovery of consciousness should be treated as status epilepticus. Treatment needs to incorporate anticonvulsant drugs (fast acting, but short duration, such as diazepam) and anti-epileptic drugs (slow onset of effect, but long acting, such as phenobarbital). The protocol recommended was:
If seizure activity persists, the patient should be anaesthetised with propofol (bolus 4mg/kg to 6mg/kg, constant rate infusion 0.1mg/kg/min to 0.6mg/kg/min). No benefit has been demonstrated in prophylactic treatment if the patient is not seizuring.
Many TBI patients have neurological deficits, leading to perturbation or loss of vital functions such as feeding, urinating or even breathing. Dedicated nursing with appropriate supportive care will avoid further complications and promote early recovery. The cornerstone is elevating the head by about 30°, without bending the neck or compressing jugular veins.
Regular bladder emptying, careful turning and skin inspection – as well as comfortable, dry bedding – contribute to normothermia and prevent pressure sores. Appropriate nutrition will help support normoglycaemia and optimum cerebral function. Normal feeding will be encouraged as much as possible, in sternal recumbency to avoid aspiration pneumonia. If necessary, a feeding tube may be placed.
Nasal tubes are best avoided to prevent sneezing, which could increase intracranial pressure. Oral hygiene is also important to reduce risk of aspiration pneumonia (chlorhexidine-based mouthwash) while moisturising the tongue.
Rehabilitation is essential to optimise recovery, and early intervention is recommended. Every patient is different in its neurological status, mobility status and expectations (pet versus working dog). Explaining the reasons for the disabilities, the animal’s progress and treatment goals at each stage of the recovery will help manage owner expectations and promote active participation in their pet’s recovery.
A multimodal approach – including manual therapies, acupuncture, physical modalities (such as therapeutic ultrasound, laser and neuromuscular electrical stimulation) and therapeutic exercises – is more likely to yield better outcomes. Manual techniques are particularly useful for motor relearning and relaxation. Indeed, the change or loss of sensory input after TBI can be very distressing for these patients. The physiotherapist may use a range of techniques depending on the effect desired, such as reducing or increasing muscle tone, proprioception training, increasing flexibility and strengthening.
The benefits of acupuncture in those cases consist mainly in balancing muscle tone and improving compensatory patterns through the fascial interconnections, and pain management through release of endorphins and serotonin.
Therapeutic exercises come in a variety of difficulty and can focus on specific tasks the patient struggles with. Therefore, each exercise needs to be tested and tailored to the patient and its stage of recovery. Hydrotherapy may be useful in the form of an underwater treadmill to promote early ambulation, increase proprioceptive input and facilitate independence. However, many TBI patients suffer from altered perception of gravity, which will not be helped by the buoyancy effect of the water. Therefore, hydrotherapy should not be used as a sole rehabilitation tool.
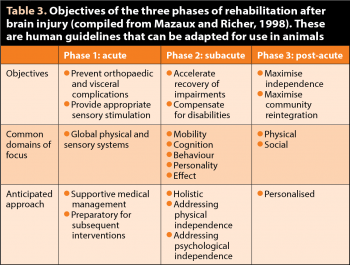
A rehabilitation programme includes pain management, return to basic functions and independence, and support to optimise quality of life. Extrapolating from human guidelines, it is divided into acute, subacute and post-acute phases (Table 3).
In the acute stage, the main goal is to limit musculoskeletal and visceral complications. This mainly means promoting normal muscle tone, often increased in TBI cases, and maintaining joint range of movement. This can be achieved via simple exercises, such as passive movements and relaxing massages. In severe cases, preventive casting of a limb in a normal position can help preserve muscle length and limit contracture, which, if left untreated, would rapidly become irreversible.
Appropriate sensory stimulation (promotion of sternal recumbency and support into standing position) is provided through short and frequent exercises during the day. Increasing sensory input by wearing a body wrap and foot wraps, for instance (Figure 1), may help manage stress and hypertonicity. Conversely, providing adequate periods of rest, with no stimulation, is very important to these patients, which can be easily overstimulated with regular checks, monitoring equipment and so on.
In the subacute period, the patient is stable or improving in neurological status, which allows the disabilities to be more precisely defined. For instance, muscle tone changes are often asymmetric, which may not be obvious initially. The initial hypertonicity may also resolve and become hypotonicity with time. The head and neck should be carefully examined for pain, muscular and fascial anomalies and joint misalignment.
The patient is assessed for ataxia, paresis and incoordination, and core stability, which can be severely affected. Balance should be precisely tested and challenged. Tests such as blindfolding or standing the patient on a wobble cushion can reveal anomalies that may not be obviousin the initial stage or in a normal, stable environment.

Once the deficits are clearly determined, a tailored rehabilitation programme is designed. At this stage, exercises such as proprioceptive training on varied surfaces; controlled assisted or unassisted standing; sitting; slow lead walking; and repeating transitions between different positions (sit to down to stand, for instance) may be useful.
If safe, exercises promoting head movement (following a treat from side to side, or up and down) will help recover proprioception and fine motor control. Again, the patient should be carefully monitored for signs of fatigue, and regular rest periods should be provided as hyperstimulation may be as detrimental as hypostimulation for long-term recovery.
In the post-acute state, the patient has recovered to a degree and the clinician will have a better understanding of the residual long-term disabilities. The goals will be to promote maximum independence and optimise the patient’s adaptation to his new life (with aids such as anti-slip floors, ramps and harnesses if necessary).
Owners have a major role to play in this part. Their dedication and bond with their pet will optimise recovery, and identify and report difficulties that may arise: a dog may walk well, but be unable to squat long enough for toileting purposes, for instance. In long-term deficits, regular aerobic activity, within tolerable range (lead walking), is recommended to improve symptoms and gradually increase exercise tolerance.
In conclusion, TBI patients are highly complex cases that require a team effort in emergency care and rehabilitation. Their almost always unique injury patterns and subsequent deficits necessitate a detailed neurological examination and tailored treatment. The prognosis is often guarded, but dogs and cats can surprise us by their abilities to recover. An adapted rehabilitation programme is essential to their recovery, even in cases that may appear minor. Long-term complications are poorly known, so these cases remain a challenge.
This BVRSMA meeting was hugely informative, with many diplomates presenting their experiences, and open for debate and discussion. We hope to see you on 3 October 2018 at our combined meeting with the behaviour group (Figure 2).