21 Jun 2016
Lyme disease is relatively new but growing threat for both pets and people in the UK. Get independent up-to-date information here to help you tackle this.

A tick feeding.

European and RCVS recognised Specialist in Veterinary Internal Medicine
Medicine Consultant, Dick White Referrals, Six Mile Bottom, Cambridgeshire, UK
Hon. Assoc. Professor of Small Animal Medicine, University of Nottingham
Lyme disease (Borreliosis) is a relatively new clinical entity caused by the spirochete Borrelia burgdorferi. It is transmitted by a variety of tick vectors depending on the geographical location, with Ixodes ricinus being the most common vector in the United Kingdom. 5–10% of dogs exposed to Borrelia develop clinical signs, which classically present as fever, with associated lethargy, followed by shifting limb lameness. These signs are not pathognomonic, and tests to document the presence of the Borrelia spirochete are needed to confirm borreliosis. Infection is usually confirmed by documenting antibodies to the C6 peptide, the constant part of the variable outer surface VIsE protein; measurable levels are present 3–5 weeks post infection and decline rapidly after treatment. Therapy with doxycycline leads to resolution of clinical signs in most cases; however, chronic non-erosive polyarthritis and glomerular nephritis are seen in chronic infections. The aetiology of these signs is not fully understood, but are likely due to Borrelia’s ability to evade the immune system and the chronic inflammatory response this evokes. Prevention is based on preventative acaricide treatments, prompt tick removal and vaccination.
Key words: Lyme disease, Borrelia, ticks, C6 peptide, polyarthritis, glomerular nephritis
Lyme disease takes its name from the town of Lyme in Connecticut, USA where the symptoms of infectious polyarthritis were first described in people in the mid 1970s. Since then the spirochete Borrelia burgdorferi sensu lato has been found to be the causal agent of Lyme disease and it has been documented to cause disease in veterinary species. Most published research relates to B.burgdorferi sensu stricto, which is the primary isolate causing disease in the USA; however, there is considerable genetic heterogenicity in B.burgdorferi species between North America and the UK. In Northern Europe isolates of Borrelia afzelii and Borrelia garinii have also been found to cause borreliosis in people.
Classic signs of canine Lyme disease follow a history of a tick bite and initially signs of fever and lethargy, followed by shifting limb lameness. Unfortunately these classic signs are not always seen and are also seen in a wide range of other disease, which can make the diagnosis of Lyme disease difficult. B.burgdorferi is also associated with glomerulonephritis and a chronic non-erosive arthritis, which are generally seen later in the course of the disease. Signs are usually seen within a month of the tick bite; however, in experimental studies disease has taken up to six months to develop.
B.burgdorferi cannot survive free in the environment and is passed between vertebrate hosts by tick vectors. In the UK the most common vector is Ixodes ricinus (also known as the sheep tick or castor bean tick); however, B.burgdorferi has also been isolated from other Ixodes species, as well as Dermacentor reticulatus (the ornate dog tick or meadow tick) and Rhipicephalus sanguineus (the brown dog tick).
Ixodes ricinus has a 2-3-year life cycle and can harbour Borrelia for most of that period. Nymph or adult ticks transmit B.burgdorferi to the host during feeding, with spirochetes multiplying within the tick before crossing into the saliva and passing into the host. It is typically reported that this process requires the tick to be attached for at least 24-48 hours for transmission to occur, and whilst, experimentally, longer tick attachment times (48-72 hours) lead to more successful transmission, studies have also shown significant rates of infection occurring within 16 hours.

Although most ticks are removed before spirochete transmission occurs, the minimum attachment time for transmission has never been definitively established (Cook 2015). When spirochetes do move in the host they divide locally within the skin at the site of infection, before disseminating. The exact mechanism by which this happens is unclear. However, it is thought that they migrate through tissue, becoming established in collagen-rich tissues such as skin and joint structures where they can survive for long periods (Krupka and Straubinger 2010).
The immune system clears most Borrelia infections before systemic signs are seen; however, in approximately 5-10% of cases, active migration then occurs through tissue to cause systemic clinical signs.
Once present, Borrelia is a persistent pathogen and evades the immune system by undergoing changes in their surface proteins and can remain undetected in skin, connective tissue and the nervous system for long periods. Clinical signs are caused by the host’s immunological response, which is often in response to a small number of spirochetes. In people, certain MHC haplotypes are more prone to severe, treatment-resistant disease, which is probably also the case in dogs.
The first documented case of canine Lyme disease in the UK was reported in 1991 (May and others 1991); however, PCR studies of ticks held at the Natural History Museum document the presence of B.burgdorferi in UK ticks back to the late 1800s, suggesting the disease has been present longer than it has been recognised (Hubbard and others 1998). The exact incidence of canine Lyme disease in the United Kingdom is largely unknown; however, studies have documented seropositivity to B.burgdorferi in dogs across the country. Seropositivity is higher in dogs living in rural areas compared to those living in urban areas, and in animals with a history of tick bites (May and others 1991). Despite high seropositivity relatively few dogs develop clinical signs. The exact proportion of seropositive animals which develop disease is unknown, but believed to be around 5-10% (Greene and others 2012). A higher proportion is seen in man with around 90% of people developing clinical signs (Littman and others 2006).
The reported incidence of Lyme disease in people is gradually increasing, rising from 0.5 cases per 100,000 of the population of England and Wales in 2001 to 1.73 cases per 100,000 in 2011. In endemic areas, such as the Highlands of Scotland the incidence is significantly higher, with 56 cases per 100,000 of the population reported in 2010 (Slack and others 2011).
VIDEO: Lyme disease can also affect people. Veterinary surgeon Sarah Bignell – who contracted Lyme Disease herself – offers a personal and exceptionally moving account of her experience living with the disease.
The current incidence of Lyme disease in dogs is unknown; however, studies show that dogs are regularly exposed to ticks carrying B.burgdorferi, with an estimated risk of a dog encountering an infected tick in the UK being around 1 in 200, over a tick season (Smith and others 2012). A recent report from the PDSA showed a 560% increase in suspected and confirmed cases seen by the PDSA since 2009 (www.pdsa.org.uk).
The increase in reported Lyme disease in people is due to many factors which include increased awareness, better diagnostic tests and reporting, but also an increasing tick population. Data from the 10th Tick Borne Disease conference hosted by Lyme Disease Action in the UK in 2011, demonstrated a threefold increase in female ticks from 1994 to 2004 and a 20-fold increase in B.burgdorferi infected ticks from 1991 to 2009 (Cook 2015). Data from Public Health England reveals 15% of cases in people were acquired abroad, which is important to consider with increasing overseas pet travel and importation. Although the incidence of B.burgdorferi infected ticks in the UK is a relatively low 2.3% (Smith and others 2012), studies in other areas have documented higher levels of infection; for example, in over 50% of I.scapularis ticks in areas of Canada (Bouchard and others 2015) and up to 47% of Ixodes ticks in parts of Switzerland (Jouda and others 2004).
In the UK 4.2% of cats are reportedly seropositive to B.burgdorferi (May and others 1994); however, none of these cats had signs associated with lameness or recent disease, thus despite their seroconversion Lyme disease has not been described as a distinct entity in feline medicine. In experimental studies cats do develop lameness, but at much high doses than dogs, suggesting cats are more resistant to the infection.
Initial signs of borreliosis are acute fever (>40˚C), shifting limb lameness and associated lethargy. There may also be joint swelling and enlargement of the local lymph nodes. These signs appear to be most severe in younger dogs and immunocompromised animals.
Lameness is usually first seen in the limb closest to the site of tick attachment and is thought to be caused be the spread of spirochetes through the skin, muscle and joint. Classically, the lameness improves over 2–3 days at which point signs may resolve completely or appear in a different limb.
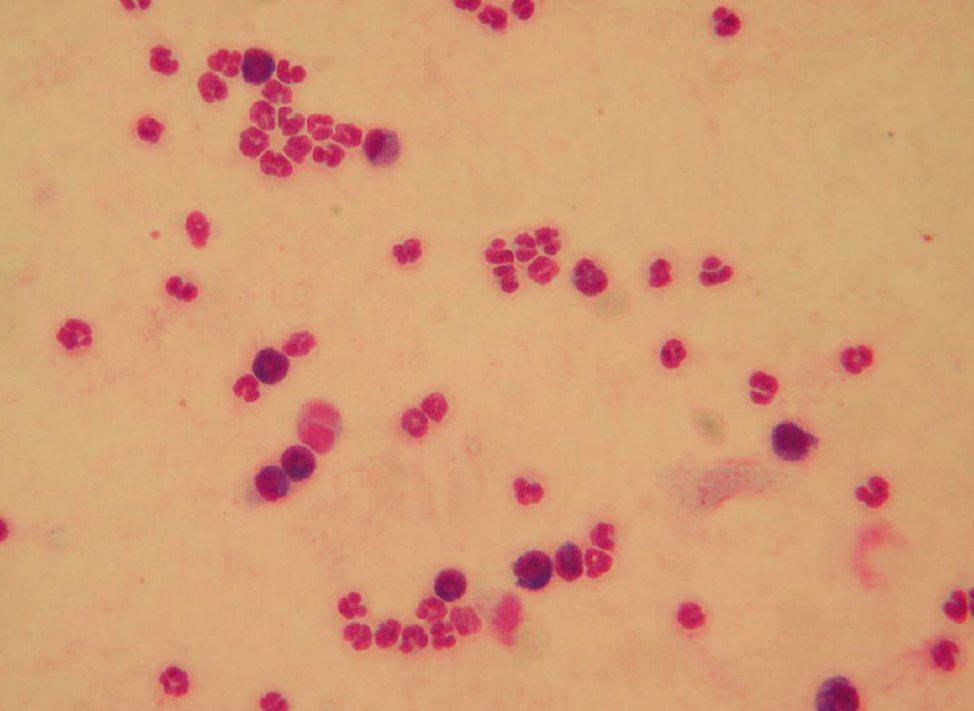
In a proportion of dogs a chronic non-erosive polyarthritis may develop, this is most likely in patients with chronic infection which has been incompletely cleared by the immune system (Figure 1) representing an immune-mediated polyarthritis.
Diagnosis of Borrelia as the trigger can be difficult; however, prolonged treatment with antibiotics and in some cases descending immunosuppressive steroids will lead to improvement in most cases.
Protein-losing nephropathy (PLN) has been documented in dogs with spontaneous Borrelia infection. This so called ‘Lyme nephropathy’ has not been documented in experimental models and the underlying pathophysiology is unclear. It has most commonly been reported in Northern America but has also been seen in the UK. Dogs develop glomerular nephritis, lymphocytic plasmacytic interstitial nephritis and tubular necrosis.
This leads to weight loss, lethargy and anorexia as a result of the PLN leading to renal failure. About half of dogs developing Lyme nephritis have a history of lameness, with the signs of PLN being the first sign of Borrelia in many cases.
In people a dramatic “bull’s eye” skin lesion called erythema chronica migrans (ECM) develops in up to 90% of people with Lyme disease. This classic bull’s eye lesion is not seen in dogs; however, a reddish rash can be seen for the first week or so after tick attachment. Neurological signs due to meningitis, encephalitis and perineuritis are seen occasionally in the later stages of infection in man. Although focal meningitis and encephalitis lesions have been documented in experimental models, neurological signs secondary to Borrelia in dogs are extremely rare. Arrhythmia secondary to Borrelia-induced myocarditis has occasionally been reported in dogs, which is similar to Lyme carditis seen in man.
As described, diagnosis of Lyme disease by clinical signs alone is challenging and is based on having appropriate clinical signs, supportive laboratory data, exclusion of other possible differential diagnoses and a positive response to treatment (Figure 2).

Haematological and biochemical changes are not pathognomonic of borreliosis, although may support the presence of an inflammatory response. Signs of leukopenia or thrombocytopenia may suggest concurrent infection with a rickettsial pathogen, such as Anaplasma phagocytophilum, as coinfection is relatively common. Regular urinalysis to monitor for PLN is suggested, with a UPC ratio being the best marker of proteinuria. Joint taps will have high numbers of non-degenerate neutrophils, with increased protein content. Joint fluid will have a reduced viscosity and should be negative on bacterial culture.
During infection, Borrelia organisms change their outer surface proteins (Osp) to allow transmission and increase their chances of survival in the host. Initially this is a change from the surface protein OspA to OspC and later OspF. The production of OspC is essential for spirochete transmission and allows it to establish infection. Another outer membrane protein, known as VIsE or variable major protein-like sequence, rapidly changes its structure after infection allowing rapidly changing antigenic variation, which leads to difficulty in the host producing antibodies which can neutralise the infection. The constant non-variable part of the VIsE, the C6 peptide, has been shown to correlate very well with the presence of B.burgdorferi infection, with measurable levels present 3–5 weeks after infection and declining rapidly after successful treatment (Wagner and others 2012). Positive serology for C6 antibodies allows rapid and definitive diagnosis of canine Lyme disease.
Occasionally, B.burgdorferi can be visualised in body fluids (e.g. synovial fluid) using dark field microscopy or in tissue after silver or immunological stains; however, the spirochete density is usually very low making diagnosis difficult by this method. Culture of Borrelia organisms is similarly difficult and, as such, not clinically applicable.
Quantitative PCR tests such as real-time PCR have revolutionised diagnostics in many areas of veterinary medicine and the same is true of canine Lyme disease. A variety of PCR tests exist; however, those with primers to plasmid DNA are more sensitive due to the multiple copies present within each bacterium. Although blood and joint fluid can be used for PCR, spirochetes tend to invade through tissue rather than passive dissemination through the bloodstream, thus tissue PCR is much more sensitive. In particular PCR of synovial membrane and skin has been shown to be much more sensitive, especially in the later stages of the disease.
Early and effective antibiotic therapy has been shown to be very effective in reducing spirochete numbers, leading to rapid improvement in arthritis signs over a 24–48 hour period. Doxycycline at 10 mg/kg SID or BID is the drug of choice for the treatment of B.burgdorferi although a number of other antimicrobials also have efficacy (Table 1).

Doxycycline is lipid soluble thus has good tissue and cellular penetration. Treatment is generally used for four weeks; however, research has shown that not all dogs will clear the infection within this period, and signs due to recrudescence of infection are occasionally seen (Straubinger and others 2000). Doxycycline should not be used in growing animals due to its deleterious, but mainly cosmetic, effects on skin, nails and tooth enamel. Although doxycycline is less likely to cause these effects compared to other tetracyclines, alternatives, such as amoxicillin, are suggested for growing animals. In the UK, all of these antibiotics are used under the cascade as there is not a licensed product for the treatment of canine Lyme disease. Doxycycline also has immunomodulatory and chondroprotective effects which are helpful in the treatment of polyarthritis (Yu and others 1992).
If proteinuria is documented (and other causes of PLN excluded) early treatment for glomerular nephritis should be instigated alongside antibiotic therapy. Angiotensin-converting enzyme (ACE) inhibitors will reduce renal protein loss through altered glomerular filtration pressure. Ultra-low aspirin therapy (0.5 mg/kg/BID) is suggested to prevent thromboembolism as a result of anti-thrombin loss and platelet dysfunction.

The best method of reducing the risk of Lyme disease is to prevent ticks attaching, or killing and removing ticks quickly when they do attach. There are several different molecules licensed. Some, such as permethrin and flumethrin, have a repellent effect against ticks while others, such as the isoxazolines: afoxolaner, fluralaner and sarolaner, are fastacting acaricides.
Regular use of an effective tick product should be suggested to all owners of dogs walked in areas with high tick numbers, especially at high risk times of the year (autumn and spring). As spirochete transmission does not occur until at least 24 hours after tick attachment, prompt removal of the ticks within this period will stop transmission of Borrelia. As any acaricide will not be 100% effective in preventing tick attachment, owner vigilance and prompt tick removal using a tick hook will further reduce risks (Figure 3).
A vaccine (Merilym 3) is also available to provide protection against borreliosis. The aim of vaccination is to induce antibody formation to the Borrelia surface proteins – such as OspA. Vaccine-induced antibodies enter the tick during feeding and bind to the bacteria in the tick gut, which is expected to reduce their migration to the tick salivary glands and thus transmission to the host. Although vaccination appears to be very effective, it is considered a non-core vaccine (Day and others 2016) and is generally only used in dogs in geographically at-risk areas and with a high degree of possible exposure (such as outdoor or hunting dogs).

 Merilym 3 contains inactivated Borrelia burgdorferi sensu lato: Borrelia garinii, Borrelia afzelii, Borrelia burgdorferi sensu stricto. Legal category: POM-V (UK); POM (Ireland).
Merilym 3 contains inactivated Borrelia burgdorferi sensu lato: Borrelia garinii, Borrelia afzelii, Borrelia burgdorferi sensu stricto. Legal category: POM-V (UK); POM (Ireland).
For further information refer to datasheet or contact Merial Animal Health Ltd, CM19 5TG, UK. ©Merial Ltd 2016. All rights reserved.
Use medicines responsibly.