11 Nov 2019
Karen Perry and Emily Hartman use an unusual case example to demonstrate their approach to management of this joint disease in cats.

Image: seregraff / Adobe stock
OA represents whole-organ failure of the synovial joint(s) and is common in cats. The progressive deterioration of one or more components of the joint is associated with pain, inflammation, peripheral and/or central sensitisation, and decreased mobility, which ultimately impact activity and quality of life. In this article, the authors use an unusual case to demonstrate their approach to feline OA management.
Diagnosis of feline OA can present a challenge; in many joints with macroscopic evidence of OA, no radiographic findings are detected and substantial debate rages regarding the significance of intra-articular mineralisations. Once a diagnosis is made, it is important to consider how progress in response to treatment will be monitored. Recent progress has been made in the assessment of chronic pain in the cat using clinical metrology instruments. These represent a practical method of assessing therapeutic efficacy in practice.
Treatment of feline OA is multimodal – including environmental and activity modulation, physical rehabilitation, dietary modulation and drug therapy with analgesic treatment classically being based on the use of NSAIDs. In many cases, however, NSAIDs are not sufficiently effective when used as monotherapy – potentially because they do not have any effect on central sensitisation, and multimodal analgesia is often necessitated.
Unfortunately, evidence for the efficacy of adjunctive analgesics in cats is scarce, but options include gabapentin, amantadine, tramadol and amitriptyline. Where a satisfactory response to treatment cannot be achieved, despite a tailored multimodal plan, surgical management may become necessary. Options include joint replacement, arthrodesis, excision arthroplasty and intra-articular fragment removal.
OA, which is a subset of degenerative joint disease (DJD), has been identified to be an important clinical disease in cats (Clarke and Bennett, 2006; Sul et al, 2014). As a disease of synovial joints, OA can be regarded as whole-organ failure (Loeser et al, 2012).
The condition is characterised by progressive degradation and loss of articular cartilage, related to altered intrinsic mechanisms of the cartilage, influenced by changes in other intra-articular tissues such as the synovium, the subchondral bone and menisci (Wei and Bai, 2016).
The progressive deterioration of one or more components of the joint is associated with pain, inflammation, peripheral and/or central sensitisation, and decreased mobility, which ultimately impact activity and quality of life (Clarke and Bennett, 2006; Gunew et al, 2008; Guillot et al, 2013; 2014; Monteiro et al, 2016).
OA is common in cats, with the prevalence of appendicular OA being estimated to range between 40% to 90% based on radiographic detection of joint changes (Clarke et al, 2005; Hardie et al, 2002; Lascelles et al, 2010a; 2012; Slingerland et al, 2011). In cats, the hip, stifle, hock and elbow joints are the most commonly affected synovial joints (Lascelles et al, 2010a).
Although much less is known about the aetiology of OA in cats as compared to dogs, idiopathic OA is considered common, with congenital, traumatic, infectious, nutritional and immune-mediated causes having been documented, similar to in other species (Lascelles et al, 2010b).
OA is considered to be incurable (Brown et al, 2008). Pain results in both local and distant deterioration of the musculoskeletal system as a result of decreased and altered mobility. The pathological processes of OA, such as joint capsule thickening and fibrosis, contribute to altered range of motion that compounds the musculoskeletal changes.
Additionally, the ongoing nociceptive input into the CNS results in somatosensory system changes and central sensitisation (Guillot et al, 2013; Knazovicky et al, 2016), which contribute to the perception of pain. The combined effects of pain, central sensitisation and activity impairment may have negative effects on the affective state, heightening anxiety, depression, sleep impairment (Knazovicky et al, 2015) and cognitive function as is reported in humans (Huang et al, 2015; Sharma et al, 2016).
Given the high prevalence of OA in cats and the severe impact on quality of life that OA-associated pain can have, it is critically important that we recognise and treat this condition. However, the difficulties in monitoring chronic pain in cats, the obstacles that complicate reaching a definitive diagnosis in many cases and the paucity of evidence supporting the majority of adjunctive therapies do make treatment of this condition a substantial challenge.
A 14-year-old spayed female domestic shorthair cat presented for assessment of reduced inclination to jump and generally decreased mobility. This had first become apparent approximately 12 months earlier and had progressively deteriorated since that time.
On presentation, a moderately stiff pelvic limb gait was noted bilaterally, with a mild weight-bearing lameness affecting the left pelvic limb. Orthopaedic examination revealed a moderate pain response on left stifle extension, circumferential thickening and synovial effusion of the left stifle. No instability of any joint was noted and the remainder of the orthopaedic examination was considered unremarkable.
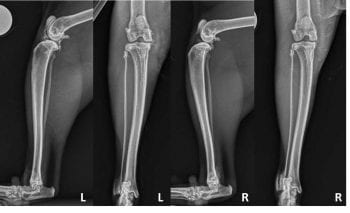
Radiographs of both stifles were taken (Figure 1). Radiographs of the left stifle revealed opacification encroachment of the infrapatellar fat pad and caudal bulging of the subgastrocnemial fascial plane consistent with synovial effusion. Several large intra-articular mineralised bodies were apparent, concentrated cranially and medially in addition to mild-to-moderate periarticular osteophytosis.
Radiographs of the right stifle revealed a small intra-articular mineralised body situated cranio-medially, but no evidence of effusion or secondary osteophytosis. Based on the clinical presentation and radiographic findings, a diagnosis of left stifle OA with concurrent osteochondromatosis was made. The right stifle was considered radiographically and clinically unaffected at this time.
While for this cat radiographic abnormalities on the left were clearly evident, this is often not the case. Indeed, in 71% of feline stifle joints with macroscopic evidence of OA, no radiographic findings are detected (Freire et al, 2011). As such, the authors could not definitively say that the right stifle was unaffected – only that they found no clinical or radiographic evidence of disease.
In the left stifle for this cat, several large intra-articular mineralisations were seen while in the right stifle, only one small area of mineralisation was noted. The question arose, what was the clinical significance of these mineralised areas? Articular mineralisations are commonly detected in radiographs of feline stifle joints (Leijon et al, 2017; Freire et al, 2010; 2011) and the prevalence of these has been reported to increase with age (Freire et al, 2010). Articular mineralisations are highly variable in size, distinct in location from osteophytes and often located in the cranial horn of the medial meniscus (Freire et al, 2010; Voss et al, 2017).
According to some authors, articular mineralisations may constitute incidental findings without clinical consequence for the cat (Allan, 2000; Mahoney, 2012; Whiting and Pool, 1985). Smaller articular mineralisations tend to be detected in the region of the cranial horn of the medial meniscus (Leijon et al, 2017; Freire et al, 2010; 2011; Voss et al, 2017), and a recent study found no association between the presence of small articular mineralisations and the severity of cartilage damage (Voss et al, 2017).
This study concluded that small articular mineralisations likely represent normal individual anatomical variation in domestic cats, similar to the situation in large non-domestic cat species (Ganey et al, 1994; Kirberger et al, 2000; Rahal et al, 2013; Walker et al, 2002). This was the authors’ conclusion for the smaller mineralised area in the right stifle – particularly as their orthopaedic examination did not reveal any abnormalities affecting this stifle and radiographs showed no evidence of synovial effusion.
The same recent study concluded larger articular mineralisations should be considered pathological and that these are associated with increased degenerative joint changes (Voss et al, 2017). These tend to be found in a different location, frequently being located between the craniomedial meniscal horn and the joint capsule or in the infra-patellar fat pad (Voss et al, 2017). This is the case for the mineralisations seen in the left stifle for the cat detailed here.
Synovial osteochondromatosis, as was diagnosed in this cat, is characterised by the formation of osteochondral nodules in the synovial lining of joints (Tas et al, 2013). Synovial osteochondromas that arise without a known predisposing cause are termed primary synovial osteochondromas, while those arising from a diseased joint are referred to as secondary synovial osteochondromas. In the secondary form, chronic OA is the major initiator of the condition (Tas et al, 2013). Synovial osteochondromatosis starts with benign reactive metaplasia of the synovium, which gives rise to the formation of osteochondral nodules in the synovial lining of the joint (Tas et al, 2013). Initially, these nodules are fibrocartilaginous and attached to the synovium by a peduncle, but as they grow, they can calcify and may break off, becoming loose joint mice (Tas et al, 2013).
Radiographic signs in cats with synovial osteochondromatosis typically include multiple nodular radiodensities of variable size within a distended joint space (Tas et al, 2013). In secondary cases, there may also be evidence of DJD, although distinguishing whether this was the primary inciting problem or alternatively a result of the intra-articular fragments is often not possible. For this cat, it was considered likely that the synovial osteochondromatosis was secondary to the development of OA, but it is impossible to be definitive regarding this.
Before commencing a treatment plan for this cat, the authors considered how to monitor progress and assess its response to treatment. Recent progress has been made in the assessment of chronic pain in the cat using owner questionnaires called clinical metrology instruments (CMIs).
The two most studied to date are the client-specific outcome measures (CSOM) and the feline musculoskeletal pain index (FMPI; Gingerich and Strobel, 2003; Lascelles et al, 2007a; 2008; 2010b; Benito et al, 2013; Gruen et al, 2015).
Over the past few years, another pain scale – the Montreal Instrument for Cat Arthritis Testing – has been developed and evaluated for completion both by owners and by veterinarians (Klinck et al, 2015; 2018a; 2018b). This scale may have potential for screening at-risk cats for the presence of OA, but further study is required.
For this cat, the FMPI was used. The FMPI is a subjective owner-completed instrument developed to assess chronic feline OA-associated pain (Benito et al, 2013). The available version of the FMPI is comprised of 17 questions with a maximum score of 68; a cat with no disease should achieve high scores (100%) while lower scores represent greater degrees of debilitation.
The questionnaire asks questions regarding the cat’s ability to complete various activities, compared with a normal adult cat without mobility impairment. It has been proven to be reliable and repeatable in both normal cats, and those suffering from OA, and is also able to distinguish between normal cats and those with OA. For this cat, the FMPI was completed by the owner at the initial appointment, with a score of 35% representing substantial debilitation.
The initial therapeutic plan for this cat involved environmental and activity modulation, physical rehabilitation, weight reduction, dietary modulation and drug therapy. Stepped access was provided to allow continued access to heights without encouraging high-impact activity.
Multiple food and water bowls were provided, and comfortable beds were made accessible. Passive range of motion – preceded by warm packing – and massage exercises were demonstrated for the owner to perform at home and physical rehabilitation appointments were made for laser therapy twice weekly. The cat had a body condition score of 6/9 and, therefore, weight loss was advised. A diet rich in omega-3 fatty acids was also advised.
The analgesic treatment of OA in cats has classically been based on the use of NSAIDs such as meloxicam (Gunew et al, 2008; Guillot et al, 2013; Lascelles et al, 2007b; Gowan et al, 2011; Benito et al, 2013).
Meloxicam is a cyclooxygenase-2 (COX-2) preferential NSAID with high oral bioavailability, efficacy, palatability and good tolerability (Lascelles et al, 2007b; Gunew et al, 2008; Guillot et al, 2013). This compound seems to improve motor activity (Guillot et al, 2013; Lascelles et al, 2007b). Meloxicam is available as tablets and an oral suspension.
A new oral, transmucosal formulation of meloxicam has been approved by the US Food and Drug Administration for the control of pain and inflammation associated with OA in dogs, but not in cats. For this cat, the decision was made to use meloxicam at a dose of 0.05mg/kg once daily for a four-week period.
After four weeks of the aforementioned treatment plan, the cat returned for reassessment. The owner reported potential very slight improvement, but no dramatic change in her activity level or stiffness. The FMPI was repeated and was consistent with this report demonstrating minimal improvement to 41%.
This is a fairly common occurrence in OA management. Despite their widespread use and obvious benefit in many cases, NSAIDs are not always sufficiently effective when used as monotherapy (Lascelles et al, 2008). While, as aforementioned, they do seem to improve motor activity, they do not seem to have any effect on central sensitisation (Guillot et al, 2013; Monteiro et al, 2016).
At this stage the authors considered some options. One would have been to trial a different NSAID in place of the meloxicam used for this cat. Robenacoxib has been shown to be well tolerated in cats with OA when given consistently for 28 days – even in patients with concurrent chronic kidney disease (King et al, 2016). Another was to trial multimodal analgesia.
The basis of this approach is to use a combination of drugs that all act at different levels of the pain pathway and, therefore, will have a synergistic effect – hopefully improving pain control and possibly allowing lower doses of individual drugs to be used, hence reducing the risk of side effects.
In this case, the authors suspected a failure to address the neuropathic component of the OA-associated pain was the reason for the suboptimal response. Therefore, an alternative NSAID was considered unlikely to result in substantial improvement. The decision was made to trial multimodal analgesia; however, this is easier said than done.
Beyond COX-inhibiting NSAIDs, treatment options for the control of pain in cats are very limited. Furthermore, evidence for the efficacy of these so-called adjunctive analgesics is extremely limited (Lascelles et al, 2008; KuKanich, 2013).
Both gabapentin and amantadine were added into the treatment regime at this stage. Gabapentin is an analogue of the neurotransmitter λ-aminobutyric acid. Gabapentin has been advocated for the treatment of neuropathic pain in cats because of experience treating neuropathic pain in humans (Backonja et al, 1998; Kukkar et al, 2013; Moore et al, 2014; Larsen et al, 2016).
Currently, while the pharmacokinetics of both oral and IV gabapentin in adult cats have been described (Siao et al, 2010), no clinical studies exist evaluating the efficacy of gabapentin in chronic pain conditions in cats.
Several case studies describing the use of gabapentin exist (Vettorato and Corletto, 2011; Lorenz et al, 2012). One case series of three cats, where gabapentin was used for treatment of musculoskeletal pain or head trauma, concluded gabapentin was of potential benefit in these cases and may provide a valuable adjunct for management of chronic pain in cats (Lorenz et al, 2012).
Another case report detailed chronic gabapentin use after traumatic incidents (Vettorato and Corletto, 2011), concluding gabapentin warrants consideration as an adjuvant for the treatment of hyperalgesia and allodynia in cats. However, in these reports, no objective or validated assessment of response occurred and the high placebo effects in owner reports may render these small case series unhelpful (Adrian et al, 2017).
Certainly, more data with larger numbers of cats is needed before definitive treatment recommendations can be made. When it is used, gabapentin is normally dosed at between 5mg/kg to 10mg/kg twice to three times daily and therapy should be withdrawn slowly. Side effects are rare, but when they do occur generally involve mild sedation and ataxia, which often resolves with reduction of the dose. For this cat, a dose of 10mg/kg was used every eight hours.
Amantadine is used both as an antiviral medication in human medicine, and for treatment of Parkinson’s due to its modulatory effects on CNS dopamine concentrations (Hubsher et al, 2012). Amantadine has also been described as an N-methyl-D-aspartate (NMDA) antagonist (Blanpied et al, 2005) resulting in its evaluation as an analgesic (Bujak-Giżycka et al, 2012).
The NMDA receptor and its ligand, glutamate, have long been implicated in the development and maintenance of central plasticity, via increased and sustained excitation of neurons, and subsequent alterations of gene and receptor expression (Latremoliere and Woolf, 2009; Baron et al, 2013). Blockade of these receptors with NMDA antagonists has been shown to both prevent the development of central plasticity, as well as treat the condition in affected animals (Wang et al, 2015; Tabakoff et al, 2016).
The use of amantadine in cats stems from anecdotal reports of efficacy (Robertson, 2008), or from demonstrated efficacy in dogs when used in conjunction with meloxicam (Lascelles et al, 2008).
While the pharmacokinetics of amantadine – administered both IV and PO – have been documented in cats (Siao et al, 2012), clinical data demonstrating efficacy is lacking. In the absence of data evaluating minimum effective concentrations, the recommendation for dosing is between 1mg/kg to 4mg/kg once daily.
As limited information is available regarding toxicity in cats, starting at the lowest dose and then increasing slowly as required based on response is recommended. In humans, the adverse effects generally include minor CNS and gastrointestinal signs.
Generally, in dogs and cats it appears to be well tolerated based on the limited information available. Amantadine is available in a liquid formulation, which is often necessary for cats as the capsule sizes are generally too large to allow appropriate dosing in these smaller patients. In this cat, a dose of 2mg/kg was used every 24 hours.
While gabapentin and amantadine were trialled in this particular case, other options could also have been considered. Tramadol is an analgesic used worldwide for its effects on improved physical function and good tolerability in humans with chronic OA pain (Schaefert et al, 2015).
The mechanisms of action of tramadol have not been fully elucidated and, to date, the majority of studies have focused on the activation of µ-opioid receptors and inhibition of monoamine reuptake as potential mechanisms (Raffa et al, 1992; Desmeules et al, 1996; Steagall et al, 2008).
The analgesic effects of tramadol are expected to be mostly related to the production of its active metabolite(s) such as O-desmethyl tramadol (M1), which binds to µ-opioid receptors with approximately 300-fold higher affinity than the parent compound (Desmeules et al, 1996; Frink et al, 1996).
However, the affinity of tramadol for the µ-opioid receptor is very low – approximately 10-fold less than that of codeine and 6,000-fold less than that of morphine. Yet, the increases in pain thresholds induced by tramadol differ from those of other opioids in that they are only partially blocked by naloxone (Desmeules et al, 1996). These latter findings indicate µ-opioid receptor activation is only one of the mechanisms of action of tramadol and M1.
Other mechanisms of action include:
These mechanisms of action can increase the activity of the endogenous inhibitory control and decrease the pain transmission likely explaining the central analgesic effects of tramadol (Monteiro et al, 2017).
Recent evidence indicated tramadol provided no clinical benefit to dogs with OA of the elbow or stifle joints (Budsberg et al, 2018). However, in cats, tramadol has high bioavailability after oral administration and M1 follows tramadol’s disposition profile (Pypendop et al, 2007; 2009).
Studies have indicated cats might have superior analgesic profile after tramadol administration when compared with dogs due to a longer elimination half-life and higher active concentrations of M1 (Pypendop et al, 2009; KuKanich and Papich, 2004).
Tramadol is a low-cost outpatient oral analgesic that is potentially a viable option for the treatment of OA pain; however, until recently, its efficacy for the treatment of feline maladaptive pain was not known. A recent study showed cats with maladaptive pain secondary to OA seem to benefit from tramadol treatment (3mg/kg twice a day) when compared to placebo treatment (Monteiro et al, 2017).
Treatment with tramadol increased weight-bearing and mobility, and decreased central sensitisation in cats with naturally occurring OA (Monteiro et al, 2017). These results were considered encouraging for promoting tramadol as a treatment for pain in osteoarthritic cats (Monteiro et al, 2017).
Another alternative that could have been considered was amitriptyline. Amitriptyline is a tricyclic antidepressant that exerts its effect by inhibiting reuptake of the neurotransmitters serotonin, norepinephrine and, to a lesser extent, dopamine (Moore et al, 2012). It has also been shown to inhibit H1 release from mast cells in vitro (Gurgel et al, 2013).
While its use in veterinary medicine has been limited primarily to behavioural disorders (Chew et al, 1998; Virga et al, 2001; Overall and Dunham, 2002), research in humans has demonstrated an analgesic effect in those suffering from interstitial cystitis – a urinary bladder disease with a chronic, neurogenic pain component (Hanno et al, 1989) – and the drug is commonly used to treat neuropathic pain (Moore et al, 2012).
Due to the similarity of interstitial cystitis in humans and idiopathic cystitis in cats, both proposed to have a neurogenic or neuropathic pain component, amitriptyline has been evaluated for efficacy in cats with idiopathic cystitis and clinical improvement was noted, suspected to be due to the efficacy of amitriptyline in treating the pain associated with the disorder (Chew et al, 1998).
The effect of amitriptyline on segmental inhibition – a physiological process that reduces the transmission of pain signals – was evaluated in 12 adult anaesthetised cats (Fromm et al, 1991).
The segmental inhibition of wide dynamic range neurons, which populate the dorsal horn and respond to all somatosensory inputs, was significantly increased by IV doses of 1mg/kg to 4mg/kg of the drug. This may be beneficial with chronic maladaptive pain states such as OA-associated pain, where amitriptyline may be able to help correct the dysfunctional inhibitory processes of the CNS that have been demonstrated in models of maladaptive pain (Adrian et al, 2017).
No data currently exist on the pharmacokinetics of amitriptyline in the cat, which make it difficult to recommend dosing. The drug’s bitter taste and potential side effects – such as reduced grooming, sedation and weight gain – may also limit its use (Chew et al, 1998). Further evidence is required before definitive treatment recommendations can be made.
The cat returned for evaluation six weeks after starting the amended treatment plan. No adverse effects from the medications had been noted. While the owner perceived some improvement in mobility, this was still not sufficient for them to accept the current quality of life as acceptable.
The orthopaedic examination and gait assessment were unchanged from previously. The FMPI was repeated and a value of 56% achieved. At this stage, options were discussed with the owner, including continued trial of alternative medications and surgical treatment options.
For cats with OA-associated pain, when a satisfactory response cannot be achieved using a combination of non-surgical therapies, surgical therapy should be considered. Surgical treatment options for cats include joint replacement, arthrodesis, excision arthroplasty and removal of intra-articular fractures via arthrotomy or arthroscopy.
When the associated clinical signs cannot be controlled with appropriate multimodal therapy, the treatment of choice for synovial osteochondromatosis is total synovectomy with removal of all loose bodies; however, this is difficult to perform completely and has never been described in cats (Flo et al, 1987). Removal of free fragments can be attempted, but many of the fragments seen radiographically may be embedded in the synovium (Tan et al, 2010), and are deceptively difficult to locate and remove.
While joint replacement forms the mainstay of surgical management of OA in dogs, commercial joint replacements are only readily available for the hip in the cat. Customised joint replacements have been performed for other joints, including the stifle – and, therefore, could have been an option for this cat – but as this would essentially have been an experimental surgery, this option was reserved for if other, less-aggressive, surgical options failed to provide a good quality of life.
Excision arthroplasty is a salvage surgical option for end-stage OA of the hip, digits and shoulder, but is not an option for the stifle. Arthrodesis is also a surgical option for cases where joint replacement is not feasible or appropriate. While arthrodesis relieves the pain associated with end-stage OA, it also obliterates motion at the joint and is therefore better suited to low motion joints, such as the carpus and tarsus. Carpal and tarsal arthrodesis have been performed in the cat as appropriate salvage options for end-stage OA (DeCamp et al, 1993; Mathews et al, 1995; Calvo et al, 2009; Fitzpatrick et al, 2013).
Shoulder arthrodesis is well tolerated, but technically challenging. Arthrodesis of the stifle and elbow result in profound gait abnormalities in dogs and careful case selection and client communication to manage expectations are critical.
Stifle arthrodesis in cats may be better tolerated than in dogs based on the authors’ experiences and a case report of two cats (Belch et al, 2012). However, this remains a salvage option only appropriate after more conservative surgical measures have been trialled.
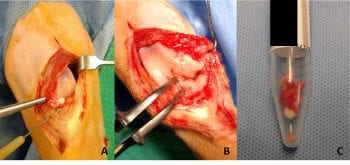
In this cat, the decision was made to proceed to surgical exploration of the stifle joint via arthrotomy, and to remove as much of the synovium and mineralised material as possible (Figure 2). The medial meniscus was found to be mineralised in addition to the infrapatellar fat pad and much of the joint capsule, and this was removed entirely along with as much of the synovium and fat pad as possible. The cranial and caudal cruciate ligaments, and lateral meniscus, were intact and palpably normal, and were left in situ.
Postoperative radiographs (Figure 3) revealed decreased mineralisation within the joint. The cat was discharged on the same doses of gabapentin, amantadine and meloxicam as aforementioned, and restricted to crate rest for a period of six weeks. After four weeks, the FMPI had improved to 72%.
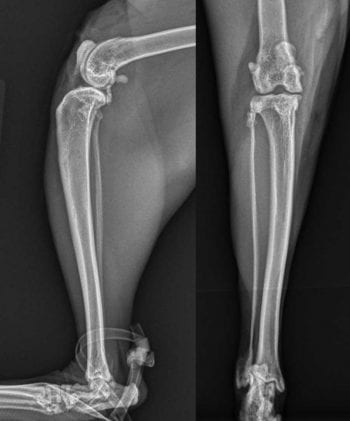
At that time no left hindlimb lameness was evident. While the left stifle remained palpably thickened relative to the right, the pain response upon stifle manipulation had resolved. The gabapentin and amantadine were stopped at this time. After a further two weeks, the FMPI had remained largely static at 75% and the meloxicam dose was gradually tapered down.
At the time of writing this report – eight months postoperatively – the owner only uses the meloxicam periodically at a dose of 0.02mg/kg when she notices reductions in the cat’s mobility. The FMPI has remained largely static, fluctuating between 75% and 80%.
This case report and the discussion around it detail some of the challenges encountered when dealing with feline OA cases – many of which stem from the limited treatment options available and the lack of evidence of efficacy for many of our adjunctive therapies.
Feline OA remains a challenging entity to treat, despite growing interest and research in this field, and an urgent need exists to develop more effective treatments for the relief of OA-associated pain. To the authors’ minds, the most promising future option being evaluated is anti-nerve growth factor (NGF) therapy.
NGF is essential for the survival of sensory and sympathetic neurons during development. However, in the adult, NGF and its interaction with tropomyosin receptor kinase A receptor (TrkA) has been found to play a critical role in nociception and nervous system plasticity in pain conditions. Therefore, various monoclonal antibody (mAb) therapies targeting this pathway have been investigated in the development of new pharamacotherapies for chronic pain.
Although none of the mAbs against NGF are yet approved for use in humans, they show promise for the effective control of pain. Recently, species-specific mAbs for the management of OA-associated pain in dogs and cats have been developed, and early clinical trials have been conducted. Anti-NGF therapy looks to be both very effective and very promising as a novel therapy against chronic pain in dogs and cats (Enomoto et al, 2019).
Abbott Laboratories (Lacey et al, 2012) and Nexvet Biopharma (Gearing, 2016) hold a patent for anti-NGF mAbs in cats. However, the only published data in cats are for the Nexvet feline anti-NGF mAb.
Initial pharmacokinetic and efficacy evaluation of frunevetmab – Nexvet’s fully felinised anti-NGF mAb – showed it had a peak plasma concentration of approximately three days and a mean plasma half-life of nine days (Gearing et al, 2016). It was well tolerated at dosages up to 28mg/kg. In placebo-controlled, unblinded work, a single dose of 2mg/kg significantly decreased subjective lameness scores compared with placebo-treatment (Gearing et al, 2016). No adverse effects were noted.
A single clinical trial has been published (Gruen et al, 2016). This was a blinded, placebo-controlled pilot study conducted in 24 cats with OA-associated pain to assess the efficacy of a single dose of frunevetmab over a nine-week period.
Outcomes were objectively measured using collar-mounted accelerometers and CMIs (Gruen et al, 2016). Accelerometry revealed a significant increase in activity compared with placebo treatment from two weeks to six weeks after injection, greater than that seen in previous studies evaluating activity increases after administration of meloxicam (Gruen et al, 2015).
Subjective owner assessments showed a significant effect of treatment over the first three weeks after administration. A larger-scale efficacy study is ongoing with a target enrolment of 250 cats; the results of this study, when available, will be a valuable contribution to the limited knowledge regarding this treatment option in cats.