22 Apr 2019
James McMurrough discusses the importance of staging this disease of the heart to provide treatment and management.

Figure 1. Myxomatous mitral valve disease is significantly over-represented in cavalier King Charles spaniels. Image: © Buraktumler / Adobe Stock
Advances in the management of myxomatous mitral valve disease are continuously appearing in the literature. Some studies have demonstrated prolongation of the pre-clinical period – highlighting the importance of early disease detection and early medical intervention – while others have focused on managing the clinical signs of heart failure and prolonging survival in those affected.
This article aims to summarise the findings of the relevant published evidence, emphasising the importance of disease staging, while providing clear guidelines on the ideal management for each stage of the disease.
Myxomatous mitral valve disease (MMVD) is the most common acquired cardiac disease in dogs, accounting for more than 70% of all cardiac disease, of which a significant proportion of cases will progress to heart failure1.
The disease is more prevalent in small to medium breeds rather than large breeds, with the cavalier King Charles spaniel (Figure 1), Chihuahua, dachshund, poodle, Pomeranian and papillon significantly over-represented. In some of these breeds, cardiac mortality significantly outweighs other causes of death.
Larger-breed dogs can also be affected; the disease progresses to heart failure more quickly in larger breeds, often with systolic dysfunction. The disease is uncommon in young dogs (other than the cavalier King Charles spaniel), but common in older dogs, with some small breeds having a prevalence of more than 90% in individuals more than 10 years of age. MMVD is more common in males than females and disease progression is also faster in males2,3.

MMVD is a progressive, degenerative process characterised by thickening, nodular distortion and contraction of the valve leaflets. These changes are commonly accompanied by valve prolapse; rupture of low order chordae tendinae, due to abnormal stress on the valve leaflets; and disease extending into the chordae. These processes ultimately lead to inadequate coaptation of the mitral valve leaflets and regurgitation of blood into the left atrium.
The severity of the mitral regurgitation will dictate the rate of progression of heart disease and the haemodynamic consequences may result in activation of compensatory mechanisms. These compensatory mechanisms include increased heart rate, increased contractility, eccentric left ventricular hypertrophy, left atrial dilation, increased circulating volume and neurohumoral activation.
Eventually, these compensatory mechanisms become insufficient to maintain adequate heart function and signs of exercise intolerance or collapse ensue, due to reduced cardiac output, as well as signs of congestive heart failure (CHF) due to increased venous pressures.
Post-capillary pulmonary hypertension may also develop in the latter stages of the disease, secondary to elevated left atrial pressures, causing clinical signs of collapse, syncope, exercise intolerance, and right-sided CHF.
In approximately 30% of cases, the tricuspid valve is also affected, leading to concurrent tricuspid regurgitation and potentially right-sided CHF4. It is worth noting, however, more than 50% of patients with MMVD have such slowly progressive disease, they never develop CHF.
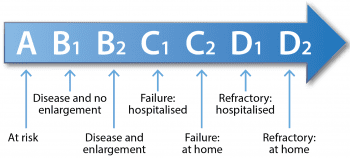
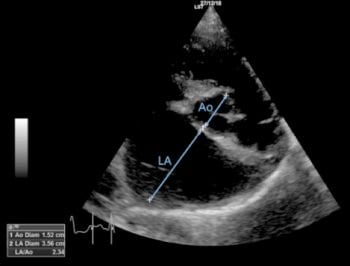
In small breed dogs, MMVD is usually chronic and slowly progressive, with many patients having pre-clinical disease for years prior to the onset of clinical signs of CHF5,6. The staging of MMVD is, therefore, very important when considering therapeutic options, as specific recommendations in the literature have been made based on heart disease stage (Tables 1 and 2).
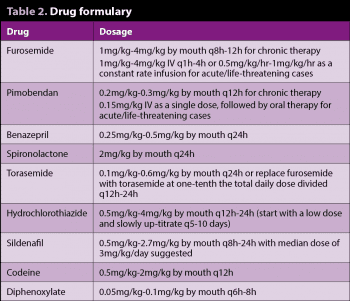
In 2009, the American College of Veterinary Internal Medicine (ACVIM) published a consensus statement on the diagnosis and treatment of chronic valvular disease1, which is now the accepted staging system (Figure 2) for chronic valvular disease – replacing the previous functional classification systems, such as the New York Heart Association system.
Stage A identifies patients at high risk for developing heart disease, but having no identifiable structural disease of the heart (for example, a cavalier King Charles spaniel without a heart murmur).
Stage B identifies patients with atrioventricular valve disease, but no clinical signs of heart failure. This stage is further subdivided into two based on the presence or absence of cardiomegaly.
Stage B1 identifies patients with atrioventricular valve disease, but no radiographic or echocardiographic evidence of cardiomegaly.
Stage B2 identifies patients with haemodynamically significant atrioventricular valve disease and radiographic or echocardiographic evidence of left-sided cardiomegaly.
Stage C identifies patients with past or current signs of CHF due to atrioventricular valve disease, and may be further subdivided into those with acute heart failure requiring hospitalisation or those with heart failure able to be treated on an out-patient basis.
Stage D identifies patients with end-stage heart failure due to atrioventricular valve disease that are refractory to “standard therapy”. These patients can also be subdivided into those requiring hospitalisation or those able to be treated as out-patients.
MMVD stage A and B1 patients have not yet developed heart disease (stage A) or else have haemodynamically stable MMVD (stage B1) and do not require medical or dietary therapy. Instead, periodic monitoring to identify disease progression is warranted; for example, yearly auscultation for patients in stage A, and 6 to 12-monthly radiography or echocardiography for patients in stage B1.
MMVD stage B2 patients are a large heterogenous group, with some on the verge of developing CHF and others who may never progress to CHF. The median time to onset of CHF in those that do progress is approximately 27 months5,6.
Pimobendan has been studied in several small animal cardiac disorders and is being used with increased frequency in canines with pre-clinical heart disease. Pimobendan is a calcium sensitiser and phosphodiesterase 3 inhibitor, which acts as an inodilator, producing both positive inotropic and vasodilatory effects without increasing myocardial oxygen demand.
The effect of pimobendan in dogs with cardiomegaly (EPIC) clinical trial7 found patients with stage B2 MMVD – which met the criteria for inclusion (Panel 1) receiving pimobendan at a dose of 0.2mg/kg to 0.3mg/kg by mouth every 12 hours – had prolongation of the pre-clinical period by a median time of 462 days (a difference of approximately 15 months) compared to the placebo group. The results of this study indicated patients suspected of having pre-clinical MMVD should be evaluated echocardiographically and that pimobendan is warranted in patients meeting the EPIC criteria.
Patients in which echocardiography is not available with a vertebral heart score of more than 11.5 would also likely benefit from pimobendan therapy, as they are likely to meet the EPIC echocardiographic criteria and progress to CHF in the next 6 to 12 months8-9,10.
Patients with stage B2 disease that do not meet the EPIC criteria do not warrant pimobendan therapy, but should be re-evaluated at 6 to 12-monthly intervals for progressive cardiac enlargement that does meet the EPIC criteria. It is questionable how much activation of the renin-angiotensin-aldosterone system (RAAS) occurs in MMVD prior to the onset of CHF and several clinical trials have tried to determine whether an angiotensin-converting enzyme inhibitor (ACEI) would be beneficial in these patients. So far, clinical trials have found ACEI therapy has had either little or no effect in delaying the onset of CHF5,6,11. Therefore, ACEIs are not recommended in MMVD prior to the onset of CHF.
A prospective clinical trial looking into the effects of benazepril and spironolactone in delaying the onset of CHF is underway – the results of which may yet change our approach to the management of pre-clinical MMVD once more.
To understand the treatment of CHF it is important to be familiar with the series of pathophysiological events that led to its development. Initially, this complex series of neurohumoral mechanisms has a beneficial effect in maintaining cardiac output; but, with time, they further perpetuate the progression of disease and lead to a worsening heart function.
The endpoints of this complex series of mechanisms are:
Although patients with stage C MMVD may have a reduction in cardiac output, which can cause signs of exercise intolerance, it is the CHF signs of backward failure, such as pulmonary oedema and ascites that are primarily responsible for the patient’s clinical signs.
The therapy for patients with stage C disease can be subdivided into acute (hospital-based) therapy and chronic (home-based) therapy.
Loop diuretics are the cornerstone of therapy for CHF as they reduce intravascular volume, therefore relieving venous congestion. Furosemide has long been accepted as the first-line loop diuretic, due to its potent action on the Na+: K+: 2Cl− cotransporter in the ascending loop of Henle. This inhibits sodium, chloride and potassium reabsorption, leading to sodium loss and, with it, water, resulting in potent diuresis.
Maintenance doses for chronic therapy are 1mg/kg to 2mg/kg by mouth every 8 to 12 hours; doses should be gradually reduced to the lowest effective dose where possible. Oral bioavailability is 77% with maximum plasma concentration being achieved within 30 minutes of dosing; however, patients with right-sided CHF and gastrointestinal wall oedema will have significantly reduced bioavailability and IV administration (at least in the initial stages of therapy) should be considered instead.
In acute CHF, doses of 1mg/kg to 4mg/kg IV every 1 to 6 hours are sometimes used while closely monitoring respiratory rate, respiratory pattern, hydration status and urine output. IV administration has the additional benefit of vasodilation, which can further reduce pre-load. IV constant rate infusion (0.5mg/kg/h to 1mg/kg/h) can be considered in cases with fulminant pulmonary oedema, although its efficacy over bolus injection varies between studies12,13-14.
Despite the widespread use of furosemide, few canine clinical trials have been published. Furosemide also requires 8 to 12-hourly dosing as its half-life is relatively short (one to two hours in dogs) and furosemide resistance can develop over time, meaning higher doses are needed to maintain diuresis1,15.
Torasemide is licensed in the UK and this medication has approximately 10 times the potency of furosemide, greater oral bioavailability (80% to 100%), and the data sheet recommends once-daily dosing (half-life eight hours in dogs)15-17. It has also been suggested torasemide may reduce afterload as it causes vasodilation, and may also reduce remodelling and improve cardiac function through anti-aldosterone effects.
Torasemide is most commonly used by cardiologists in refractory CHF patients experiencing furosemide resistance. However, the Test Study found torasemide to be “non-inferior” to furosemide when used as a first-line diuretic for stage C MMVD18. The Toric Study found a reduction in morbidity and mortality in human patients with chronic CHF treated with torasemide when compared to other diuretics19. It is, therefore, reasonable to consider torasemide at a dose of 0.1mg/kg to 0.6mg/kg every 24 hours as an alternative to furosemide as a first-line diuretic for CHF.
Patients receiving loop diuretics should have their urea, creatinine and electrolytes checked during the initiation of therapy and one week after dose increases, to monitor for the development of azotaemia, hyponatraemia, hypokalaemia and hypochloraemia.
Clear survival benefits have been shown with the use of pimobendan in stage C MMVD and it is recommended in all stage C patients1,20,21. Patients with chronic CHF and significant financial restrictions should have pimobendan prescribed in preference to an ACEI following the results of the Quest Study20. Patients with acute CHF that will not tolerate oral medication, due to the severity of their dyspnoea, can be given a single IV bolus of pimobendan at 0.15mg/kg followed 12 hours later by the oral preparation.
Multiple studies have also demonstrated a survival benefit from the addition of an ACEI to conventional therapy for CHF and an ACEI is recommended in all home-treated stage C patients22,23,24,25. However, no consensus exists on the addition of an ACEI to standard therapy in hospital-treated CHF patients, although many cardiologists would consider its use1. When prescribed in the acute situation systolic blood pressure should be closely monitored for the development of significant hypotension. Patients started on an ACEI should have their urea, creatinine, potassium and blood pressure monitored in the initial stages of therapy.
Spironolactone is reserved for patients with CHF and is used because of its anti-aldosterone effects and possible anti-fibrotic effects, rather than its weak diuretic effects. Spironolactone has been shown to increase survival times in dogs with CHF and is well-tolerated long term26,27.
It is worth noting that, despite individual evidence for the use of each drug, no prospective study has looked at the use of pimobendan, an ACEI and spironolactone in combination with furosemide for the treatment of CHF – despite this being the standard combination (sometimes referred to as quad therapy) recommended by most cardiologists for stage C MMVD.
Advanced heart failure is challenging to manage as very few clinical trials or studies have been carried out defining exactly how we classify or optimally treat this stage of disease, and many of these cases may benefit from referral to a cardiologist. Despite this, survival times in advanced heart failure can be surprisingly long, with a median of nine months described in one study28. The ACVIM consensus statement classified this stage as patient’s refractory to “standard therapy” (as outlined previously for stage C) or patients with a lack of response to furosemide at doses of more than 6mg/kg to 8mg/kg per day.
One of the main reasons why patients become refractory to standard therapy is diuretic resistance. This occurs due to neurohumoral activation, enhanced sodium and solute reabsorption in the proximal convoluted tubule, nephron hypertrophy and decreased renal responsiveness to natriuretic peptides.
Given the relative lack of evidence in stage D dogs, a sensible approach may be to use additional therapy (such as sequential diuretic therapy) or consider switching the furosemide to torasemide, so long as severe renal dysfunction is not present.
As in the Test Study, an initial torasemide dose of 0.2mg/kg every 24 hours should be used and titrated upwards in 0.1mg/kg increments as needed. Alternatively, one can initiate the daily total torasemide dose at one-tenth the daily total furosemide dose, up to a maximum dose of 0.6mg/kg/day. The total torasemide dose can be administered once daily or divided into two equal doses 12 hours apart.
Hydrochlorothiazide is a thiazide diuretic that inhibits the resorption of sodium and chloride in the distal convoluted tubule, leading to diuresis through excess sodium excretion. It should always be used in combination with a loop diuretic. Hydrochlorothiazide can be prescribed at 0.5mg/kg to 4mg/kg by mouth every 12 to 24 hours, with the recommendation to start at the low end of the dose range and up-titrate the dose every 5 to 10 days to effect.
Close monitoring of urea, creatinine and electrolytes are essential as acute kidney injury is a possible complication of therapy with hydrochlorothiazide.
Stage D patients may also benefit from draining of effusions to improve comfort, and anxiolysis when dyspnoeic. Optimisation of the dose of pimobendan, ACEI and spironolactone should also be considered in stage D, as well as therapy for concurrent arrhythmias with deleterious cardiovascular effects (for example, atrial fibrillation).
In-hospital management of stage D MMVD is beyond the scope of this article and best managed by experienced cardiologists.
A cough does not accurately predict the presence of CHF in patients with MMVD29. A cough is more likely to be associated with airway compression secondary to cardiomegaly or concurrent airway disease (for example, chronic bronchitis) than pulmonary oedema in MMVD patients. A cough may improve on pimobendan therapy, as the left side of the heart may decrease in size, but those with chronic airway compression will continue to cough. When certain pulmonary oedema is not present, these patients may benefit from a cough suppressant such as codeine phosphate or diphenoxylate.
Two studies have suggested resting respiratory rates (RRRs) represent a useful objective clinical variable to identify dogs with left-sided CHF30,31. Owners should be educated on the technique (visit https://cardiaceducationgroup.org) and importance of monitoring RRRs, as those consistently fewer than 30 breaths per minute (bpm) are consentient with good control of CHF; more than 30bpm shows inadequate control, and more than 40bpm indicates a need to attend the clinic for further evaluation.
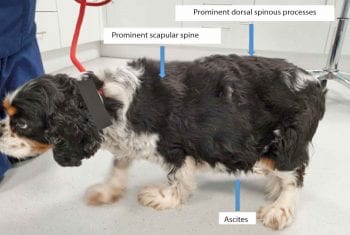
Despite some benefits having been shown from feeding a cardiac diet in the short term to patients with early MMVD, the 2009 consensus does not recommend dietary therapy at this stage1,32.
Cardiac cachexia (Figure 5) is a serious complication of advanced heart disease, and has been associated with a poorer prognosis in many human and canine studies34-35. As a result, patients with more advanced heart disease (stages B2 to D) should be fed a varied, highly palatable, high-calorie and high-protein diet to help maintain normal bodily condition. Patients should be monitored with regular weighing and body condition scoring. Those with advanced cardiac disease should also be fed a moderately sodium-restricted diet and avoid high salt content treats.
Supplementation with omega-3 fatty acids has been shown to increase appetite in patients with heart failure and can be recommended to patients if no history of coagulopathies or pancreatitis has been noted36.
Pulmonary hypertension is a common complication of MMVD (most commonly in stages C and D), and moderate to severe pulmonary hypertension has been associated with a poorer outcome37,38. Treatment of severe pulmonary hypertension confirmed by echocardiography should initially be based on optimisation of CHF control as discussed previously.
Those patients with clinical signs of severe pulmonary hypertension (such as exercise intolerance, collapse or syncope), despite optimal CHF therapy, should be treated with the phosphodiesterase V inhibitor sildenafil citrate. Although, in studies, sildenafil has not consistently reduced pulmonary arterial pressures, it has been shown to significantly improve clinical status in dogs with pulmonary hypertension39-41.
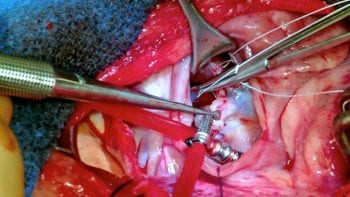
While surgery is commonly performed in humans with degenerative valvular disease, it is still regarded as a novel therapy within the veterinary profession – available at very few centres worldwide and at considerable cost. Valve repair was first described in the veterinary literature by Kanemoto et al42. Since that time, case reports43 and a case series44 have appeared sporadically, until another highly specialised team from Japan demonstrated consistently excellent results in a large series of dogs, with 93% survival at three years post-surgery45,46.
The procedure is available in the UK at the RVC Queen Mother Hospital for Animals (Figure 6), where increasing experience in performing the procedure during the past few years has led to survival and discharge rates of 100% for stage B2, and 87% for stage C patients (unpublished data).
Patients most suitable for mitral valve surgery are those in late stage B2, or early to mid-stage C with well-controlled CHF with no significant, uncontrolled co-morbidities and, ideally, less than or equal to 10 to 11 years of age. Other patients, however, are considered on discussion with the team. The cost of the procedure is capped at £17,500 and waiting times are in the order of five months (at time of writing).