27 Nov 2017
Katarina Varjonen and Tim Nuttall bring readers up to speed with the latest terminology, pathogenesis and treatment for this chronic skin disease.

Increased understanding of the complex nature of atopic dermatitis has, in turn, improved our understanding of how we can better address the clinical needs patients suffering from it show.
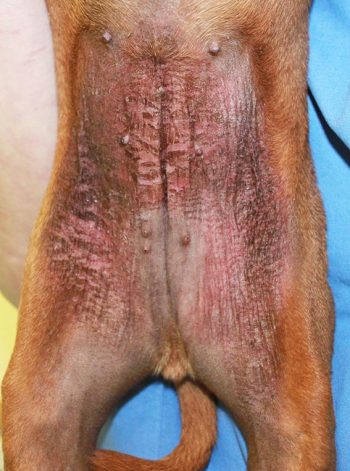
Atopic dermatitis (AD) is a common chronic disease in small animal patients. In dogs, the disease is known to closely mimic atopic eczema in people. The pathogenesis of AD is not entirely clear, and both environmental and genetic factors contribute to the development of clinical disease. Sensitisation to allergens, and subsequent inflammation and production of inflammatory mediators, is seen. Additionally, an impaired skin barrier and microbial populations on the surface of the skin contribute to disease. A defective skin barrier in AD is associated with changes in lipids and dryness, but impaired function can also allow penetrance of allergens, increasing sensitisation.
Treatment of atopic individuals aims to control microbial populations, improve the skin barrier, and control itch and inflammation. Many patients benefit from a multimodal approach to treatment, and this frequently allows reduction of medication dosages of drugs that pose a higher risk of causing side effects to the patient.
This article reviews literature on AD – with the aim of particularly looking at studies published over the past couple of years. Newer treatment options that can help manage atopic disease, as well as recent and ongoing research, are presented.
Atopic dermatitis (AD) is a common disease in canine and feline patients. Over the past few years, understanding of the disease has evolved, and treatments have been assessed and added to our choices.
This article aims to update clinicians on thoughts regarding the pathogenesis of AD, as well as give examples of ongoing research areas. Additionally, more recent treatments will be discussed. For colleagues looking for more information, free access to diagnosis and treatment guidelines, as well as review articles on the disease, visit http://icada.org
The increased understanding of the complex nature of AD that has evolved has, in turn, improved our understanding of how we can better address the clinical needs patients suffering from AD show. Desensitisation remains the only known disease modifying treatment available, but symptomatic treatment of AD has rapidly changed from very basic management using corticosteroids, to a more diverse and multifaceted approach. Although management of pruritus remains a major goal when treating the disease, we learned preventing chronic inflammatory changes and working on barrier improvement by reduction of microbial populations, as well as moisturising, allows us to gain better control of the disease.
With increased understanding of the disease, the definition of AD and the terminology used to describe “atopic” patients has slightly changed. It will likely change more once further information is obtained – particularly in felines where the pathogenesis of the disease is poorly understood.
Canine AD (CAD) is described as a “genetically predisposed inflammatory and pruritic allergic skin disease with characteristic clinical features most commonly associated with IgE towards environmental allergens”1. The latter part of the sentence emphasises not all dogs with AD show allergen-specific IgE towards environmental allergens. Dogs diagnosed with AD – where food reactions have been ruled out through a dietary trial, and do not show increased allergen-specific IgE levels towards environmental allergens – are considered diagnosed with “atopic-like dermatitis”.
Additionally, the term food-induced AD (FIAD) has been introduced. FIAD aims to describe dogs that present with clinical signs consistent with AD that fully or partially respond to dietary adjustment. FIAD is a form of a cutaneous adverse food reaction, but it should be emphasised other clinical forms exist beyond FIAD. The diagnosis of a dietary sensitisation relies on a dietary trial. Due to their low positive predictive value, serology tests cannot be used for diagnosis2.
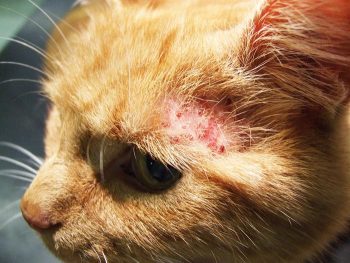
Multiple terms have been used to describe hypersensitivity reactions in cats. The International Committee on Allergic Diseases of Animals has recommended the term feline hypersensitivity syndrome is used to describe pruritic hypersensitivity conditions in cats. The term includes reactions caused by both environmental and food hypersensitivities.
For the purpose of this article, AD will be used to describe cases where reactions to dietary allergens have been excluded.
The pathogenesis of CAD is not yet fully understood, but the disease is considered closely similar to human AD. AD is a multifactorial disease, with complex interactions between genetic, environmental, microbiological, allergic, inflammatory and psychogenic factors seen3.
A heritability study conducted in British guide dogs estimated a heritability of almost 50%4. While the disease is more prevalent in some breeds than others, it has become increasingly evident the genetic background is diverse, and both immunological and skin barrier genetic abnormalities are likely involved5. A defective skin barrier, through either genetic or environmental factors, predisposes the skin to penetration by external potential allergens and microbes3,6.
Genetic defects in skin barrier proteins – such as filaggrin, a natural moisturiser – have been recognised to increase the risk of AD development in humans7.
To date, clear correlations to specific genetic mutations have been difficult to verify in dogs and, although altered filaggrin expression has been shown in an experimental model ofatopic dogs, it is possible this alteration is secondary to inflammation, rather than a primary genetic abnormality8.
Data has suggested genetic changes vary between breeds and regions, and some candidate genes in the dog have been identified when only one breed has been assessed5. One example is an area located on chromosome 27 identified as altered in atopic German shepherd dogs in Sweden. The area identified encodes a protein that is a component of desmosomes and corneodesmosomes, and mutations in this protein can, therefore, affect the skin barrier function; thereby, potentially predisposing dogs carrying the mutation to AD development9,10.
An aberrant immune response plays a key role in AD, with complex interactions between the innate and adaptive immune system seen. The over-expressed inflammatory mediators have shown to cause disruption to the epidermal barrier, but also to be major direct contributors to itch, such as interleukin 31 (IL-31). While traditional symptomatic treatment for managing AD used in the dog, as well as the cat, primarily aims to suppress inflammation, more recent research is focused on addressing specific components of the immune response.
This aims to improve control of clinical signs and, by providing a more targeted approach, reduce treatment side effects. The caninised monoclonal antibody targeting IL-31 – lokivetmab – represents this approach.
The role of Igs in AD remains somewhat unclear. Sensitisation to environmental allergens – such as dust mites, pollens and moulds – are seen in atopic dogs and is a major feature of the disease. Whether IgG plays a role in the pathogenesis of the disease is unclear, and measurements of allergen-specific IgG levels of any subtype cannot be used to show sensitisation11. Increased levels of allergen-specific IgE can also be found in dogs without evidence of AD and, although IgE is considered to play a role in the disease, diagnosis of environmental sensitisation cannot be done based on allergy testing alone. AD remains a clinical diagnosis based on exclusion of other diseases with a similar clinical presentation11,12. Although IgE does appear to play a role in feline AD, very limited information exists in the species13.
Investigations into the skin microbiome’s ability to regulate the immune system is an increasing area of research in both veterinary and human dermatology. While the host’s immune system is able to shape the skin microbiota, skin microbes also appear able to influence the development of skin immunity14. Disease flares in AD in dogs have been associated with shifts in the microbiota of the skin showing reduced diversity of the flora, with increased numbers of Staphylococcus species – particularly Staphylococcus pseudintermedius – and Corynebacterium species seen15. Similar shifts are seen in human AD patients with increases in Staphylococcus aureus, and cats with AD have also been shown to carry higher proportions of staphylococci compared to healthy cats16,17.
Antibiotic treatment of atopic patients with bacterial infections restores bacterial diversity to levels comparable to non-atopic dogs temporarily, but a shift back is rapidly seen once treatment is discontinued18. The fungal microbiota has also been assessed in healthy and atopic dogs and cats. Cladosporium and Alternaria species were most abundant on the skin of healthy dogs and cats with a reduced fungal diversity in atopic individuals19,20.
In humans, AD treatments increase bacterial diversity and resolution of disease flares have been shown to be preceded by restoration of microbial diversity16. Emollients containing non-pathogenic bacteria shown to be able to suppress growth of Staphylococcus aureus in the laboratory have reached clinical trials for treatment of atopic lesions in people21.
Limited new information exists on treatments for atopic cats. One study assessed oclacitinib, evaluating pruritic response, ease of administration and drug tolerability22. Cats were initially medicated twice daily for two weeks then once daily for an additional two weeks, using a mean dosage of 0.47mg/kg.
A total of 5 out of 12 cats assessed showed good clinical response based on validated lesional and pruritus score assessments. Remaining cats were either unchanged, worsened or discontinued medication due to treatment failure. Oclacitinib is not licensed for cats, and data on the optimal dosing and safety are not available. Lokivetmab is adapted to dogs to reduce the risk of an immune reaction towards the drug, and should, therefore, not be used in cats.
Over the past four to five years, some new treatment options have been assessed in dogs. Allergen-specific immunotherapy remains the only treatment available that can modify AD, and remains a cornerstone in treatment, with improvements seen in around 65% of patients. SC immunotherapy has traditionally been used, while, more recently, sublingual immunotherapy (SLIT) has become widely used. Controlled studies are lacking, although two uncontrolled open pilot studies showed improvement following treatment in the majority of dogs23,24. SLIT has also been assessed as a potential treatment for adverse food reactions, with promising initial data25. Intralymphatic immunotherapy for environmental allergens is also being evaluated, and is an exciting area due to promising human results for treating atopic rhinitis26,27.
Oclacitinib – a selective Janus kinase (JAK) inhibitor primarily affecting JAK1 – has been used for a few years. The drug shows good short-term safety information, while long-term safety data is not yet available28. Effect on pruritus reduction is rapid, and a similar clinical response in pruritus reduction is seen when the drug is compared to prednisolone at four hours after medication29. When compared to ciclosporin, the drug has a markedly more rapid clinical effect, with equal clinical control seen after three months of treatment30.
Lokivetmab is a recent addition to European practitioners. It is a monoclonal antibody that binds IL-31 – a potent pruritogenic cytokine31. In a mouse study, IL-31 has also been shown to affect the skin barrier and increase skin proliferation32. A major advantage of the drug is it appears safe to combine with other immunomodulatory medications and can, therefore, provide a very helpful additional option to control pruritus. The drug is registered to be used as four-weekly SC injections.
Essential fatty acid supplementation was shown to enable reduction of corticosteroid dosages with maintained pruritus control in AD patients some years ago33. A similar study was published using ciclosporin as the baseline treatment. A reduction in ciclosporin dosing – from a median dose of 4.1mg/kg/day to 2.6mg/kg/day in the treated group, and 3.8mg/kg/day to 3.3mg/kg/day in the placebo group – was seen during the study34. This suggests essential fatty acids have a ciclosporin-sparing effect.
Another study compared the drug-sparing effect of Lactobacillus paracasei K71 with that of cetirizine. The lactobacilli-treated group was able to reduce baseline medication more than the antihistamine-treated group, supporting an apparent ciclosporin and corticosteroid-sparing effect from the probiotic35.
Topical spot-ons aimed at improving skin barrier function have also been assessed, with the benefit in clinical control seen in some studies, while a double-blinded controlled study was unable to differentiate the placebo group from the medicated one36,37. This may reflect a difference between formulations or study design.
The anti-inflammatory effects of PEA appear to be mainly due to its ability to modulate mast cell activation and degranulation38. Ultramicronised PEA was assessed for treatment of AD in dogs, with improvement in pruritic and lesional scores seen. Based on this open-label study, the product improved clinical signs in 80% of dogs that completed the eight-week study, and few side effects were seen, with 7% reporting gastrointestinal upset40. Further studies are ongoing. Some support for the benefit of zinc methionine supplementation to atopic patients on ciclosporin – or particularly, glucocorticoid medication – has also been shown41.
The past few years have increased our understanding of atopic disease and added some options to our treatments. AD remains a challenging disease for both owners and vets, and treatments need to be individualised to achieve the best clinical outcome.