5 Jun 2017
Silke Salavati describes how chronic inflammatory conditions of the gastrointestinal tract develop in cats, plus treatment methods.
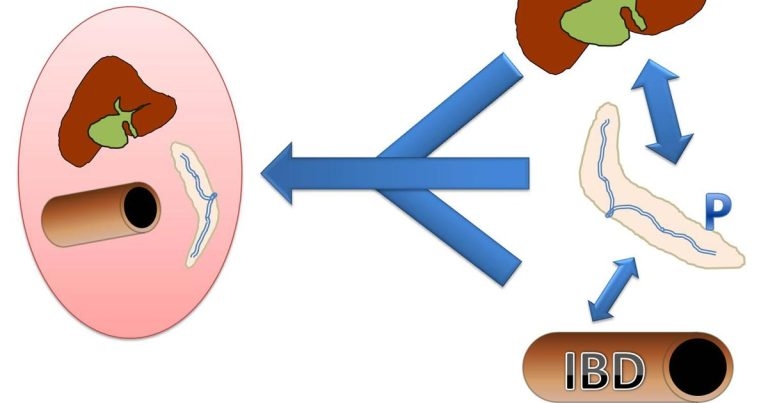
Figure 2. The relationship and co-occurance of feline cholangitis (C), pancreatitis (P), inflammatory bowel disease (IBD) and triaditis.
When it comes to chronic conditions of the gastrointestinal (GI) tract and associated structures, cats frequently suffer from concurrent inflammatory disorders, such as cholangitis, pancreatitis and inflammatory bowel disease (IBD) – collectively termed triaditis. The most important differential diagnosis for IBD in cats is intestinal small cellular lymphoma. Both the diagnosis of IBD and GI lymphoma should alert the clinician to the possibility of hypocobalaminaemia presence (or vice versa), to which cats seem to be particularly prone. This article aims to highlight novel aspects of terminology and pathogenesis of inflammatory GI disorders, and give an overview of new aspects of treatment, including the use of probiotics.
Differential diagnosis for chronic gastrointestinal (GI) disorders in cats is as extensive as in dogs and must not only include intra-GI causes, but a variety of extra GI causes – particularly renal, pancreatic, hepatic and endocrine diseases (Figure 1).
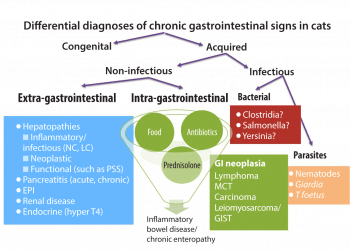
Infectious causes should also be considered – in particular, large intestinal signs should prompt investigations into Tritrichomonas foetus infection1. The presence of jaundice should alert to the possibility of acute or chronic neutrophilic or lymphoplasmacytic cholangitis (where feline infectious peritonitis remains an important differential diagnosis for the latter), or other causes of extra-hepatic bile duct obstruction (EHBO). This can include pancreatitis (even though the acute form is less common in cats than in dogs) cholangitis or neoplasia of the biliary tract and surrounding structures.
Hypocobalaminaemia is common in pancreatic diseases, such as chronic pancreatitis and exocrine pancreatic insufficiency – which is also likely underdiagnosed in cats2 – and chronic intestinal diseases. Evidence shows subnormal cobalamin availability in cats with GI disease may play a significant role in reducing the effectiveness of medical therapy3.
In this article, the relationship and occurrence of these inflammatory GI disorders will be discussed, new classifications and pathogeneses will be highlighted, and treatment options detailed.
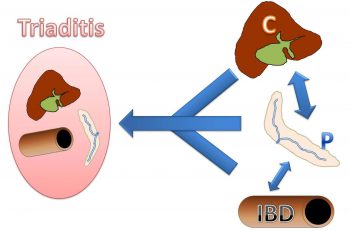
Feline inflammatory GI disease is a collective term applied to a group of multi-organ inflammatory disorders characterised by persistent or recurrent GI signs with inflammation of the liver, pancreas and/or small intestine4. This ill-defined disease entity is also often termed triaditis. Multi-organ inflammation seems more common in cats than dogs, but inflammatory conditions of single organs similar to those of dogs (such as cholangitis/hepatitis, pancreatitis and idiopathic inflammation of the intestine) also exist.
A clear body of evidence separating these single diseases from versions of triaditis or cleanly elucidating where they overlap is lacking. It seems logical a continuum of single organ to multi-organ inflammation exists (Figure 2), with the combination of pancreatitis and cholangitis slightly more common and IBD more frequently occurring without involvement of other organs5,6.
The mechanisms for triaditis development might be related to the unique pancreaticobiliary anatomy of the cat. The feline pancreatic duct enters the common bile duct before it opens into the duodenum. Also, the accessory pancreatic duct is small and only present in around 20% of cats4,7. The naturally high(er) number of bacteria in the feline duodenum, or retrograde ejection of bile up into the pancreatic and common bile ducts during vomiting, could increase the risk for pancreatitis/cholangitis7. Some evidence exists that haematogenous spread is less likely than translocation from the intestinal system in these diseases8.
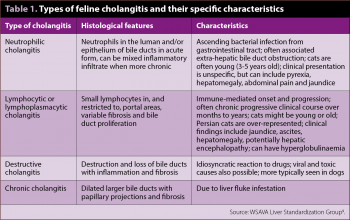
The WSAVA Liver Standardization Group has revised the histopathological classification of feline liver disease and suggested the term cholangitis, rather than cholangiohepatitis, because disruption of the limiting plate/involvement of hepatic parenchyma is not a consistent feature and, when present, is an extension of primary cholangitis9. Using this grading scheme, four distinct forms of feline cholangitis are recognised (Table 1). Distinction among the different types of cholangitis has to be ultimately based on hepatic biopsy as they share many overlapping features4.
Fluorescent in-situ hybridisation can be used to detect bacteria in-situ and complement aerobic, and anaerobic culture, of liver tissue or bile. The latter – obtained via ultrasound-guided percutaneous cholecystocentesis – is preferred, as biliary cultures are more likely to reveal pathogens (75% versus 33%) and less likely to yield contaminants (4% versus 29%) than culture from hepatic biopsies4. A publication has also showed a combination of bile culture and cytology is ideal to improve diagnostic yield10.
Treatment of cholangitis is both specific and supportive4, with treatment strategies being largely empirical, and the optimal protocol is unknown. Antibiotics are the mainstay of treatment in neutrophilic cholangitis, which should always be administered according to a sensitivity profile. If this is not available (yet), first-choice antibiotics include potentiated amoxicillin or a fluoroquinolone with metronidazole4, as most commonly isolated pathogens include Escherichia coli, Enterococcus species and clostridia4,8,11.
The primary treatment of cats with lymphoplasmacytic cholangitis involves immunosuppressive dosages of steroids until biochemical remission, followed by slow tapering over six to eight weeks to the lowest effective dose4. It is recommended antibiotics are used concurrently for two to four weeks – given the possibility of bacterial infections complicating the disease4. Nutritional support is also crucial and should ideally be ensured by oesophagostomy or percutaneous endoscopic gastrostomy feeding tubes.
The prognosis in cats with neutrophilic cholangitis is generally good, with median survival times of 29.3 months being reported4. Some report lymphocytic cholangitis had a better response when treated with ursodeoxycholic acid than with steroids4. Cats with EHBO have a guarded to poor prognosis, which improves with time if they survive surgery. Prognostic factors for cats with multi-organ inflammatory GI disease have not been identified.
Feline pancreatitis has been recognised to occur more commonly than previously thought, which is mainly because the clinical signs are vague and non-specific. Histologically, it is categorised into acute necrotising, acute suppurative and chronic non-suppurative pancreatitis4. Few studies have investigated feline pancreatitis for concurrent diseases; however, it seems it occurs more frequently with cholangitis than IBD6,7,12.
In one postmortem study, 92% of cats also had lymphocellular cholangitis, hepatic lipidosis or diabetes mellitus12, and two studies investigating triaditis showed 50% and 65% of cats with cholangitis also had pancreatitis5,6. Classical signs of pancreatitis as seen in dogs are often missing in cats, and haematology and serum biochemistry are of little diagnostic use (hypercholesterolaemia in up to 72% of cats4,13, hypocalcaemia in up to 65%14). Hence, the combination of abdominal ultrasound (which has a variable sensitivity of 24% to 67% on its own4) with assays for feline pancreatic lipase immunoreactivity, which has a sensitivity of 54% to 100%, should be used4,15,16.
Because most cases of acute pancreatitis in cats are idiopathic, supportive treatment is the mainstay of treatment. Fluid therapy should be initiated promptly, as it is likely to improve microcirculation17. Buprenorphine or butorphanol have good efficacy as analgesics. Antiemetics and gastroprotectants should be given as appropriate. Enteral feeding should be initiated early. It is not necessary to insist on low-fat diets in cats with pancreatitis; therefore, higher-calorie diets, which can be fed through feeding tubes, are acceptable4.
The treatment of chronic pancreatitis is not straightforward. Serum cobalamin concentrations should be assessed in cats with vague GI signs or inappetence3. As recurrent chronic pancreatitis can lead to exocrine pancreatic insufficiency, feline trypsin-like immunoreactivity should also be assessed – especially if hypocobalaminaemia is also present (intrinsic factor for cobalamin absorption is exclusively produced in the pancreas in cats)4. Steroids have long been thought to be contraindicated in pancreatitis, but could be beneficial in cases of chronic pancreatitis, as a degree of autoimmune inflammation is suspected4. Care must be taken if subclinical Toxoplasma infection is present, which can be “activated” with prednisolone treatment.
Similar to dogs, IBD is a diagnosis of exclusion. Its pathogenesis is likely multifactorial, with the intestinal microbiota, the local immune system and dietary antigens playing a role. A genetic predisposition has not been identified in cats, which is somewhat different to dogs and people. The primary cytokine profile from biopsies of cats with IBD is of a predominant T helper cell 1 (Th1) response, involving interleukin (IL)-1, IL-8 and IL-1211,18.
A publication has suggested the possibility of some IL-17 involvement on the gene expression (messenger RNA) level, but this has not been confirmed on the protein level and does not seem to fit with the histological phenotype of lymphoplasmacellular infiltration, as IL-17 is normally found in neutrophilic or granulocytic inflammation, such as Crohn’s disease in humans18.
Clear evidence exists to show microbiota play an important role in the development of IBD in cats. One study revealed a direct correlation of the numbers of mucosa-associated Enterobacteriaceae, as well as E coli and Clostridium species, with the amount of Th1-type cytokines found in the same biopsies, as well as clinical severity and histological changes11. Importantly, growing evidence suggests relying solely on the number of infiltrating lymphocytes and plasma cells in the lamina propria may not be helpful, as more subtle, architectural changes of the mucosa might be more suited to grade the severity of disease11,19.
Awareness is increasing that intestinal small cellular lymphoma in cats can clinically mimic moderate to severe IBD and that tests up to the point of biopsy sampling can yield the exact same results, including vitamin deficiencies, such as hypocobalaminaemia (in these cases, a marker of malabsorption and/or possible protein loss)4. Many cats with either IBD or lymphoma will have the ileum affected, so taking biopsies from this site, in addition to other parts of the intestine, is imperative to make a diagnosis20. In some cases of lymphoma, mesenteric lymph nodes will be enlarged and can be sampled by fine needle aspirates, but this is not always the case.
In most cases, histology for IBD shows a variable degree of infiltration with lymphocytes and plasma cells, as well as architectural changes, such as villous atrophy and villous fusion. However, in individual cases, the distinction from small cellular lymphoma can be impossible – even based on standard histology. Additional tools to help differentiate between small cellular lymphoma and IBD are immunohistochemistry for B and T cells (small cellular lymphomas are mostly of T-cell type) and PCR for antigen receptor rearrangement4,21. Eventually, making this distinction might be of little clinical relevance, as both severe IBD and intestinal small cellular lymphoma respond reasonably well to immunosuppressive treatments, and are associated with a fairly good prognosis22.
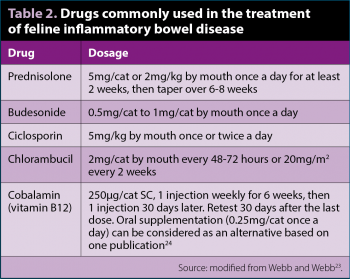
The treatment of IBD in cats should follow a similar sequential pattern than in dogs, starting with an elimination diet (single protein or hydrolysed). It has been shown up to 50% of cats with IBD respond within seven days to this treatment4. If there is no response, tylosin or metronidazole – most often in combination with steroids – should be used. Steroids alone at immunosuppressive doses (Table 2; for at least two weeks before starting to taper) can lead to complete clinical responses4.
Other immunosuppressive drugs can be used in addition to, or instead of, prednisolone if the clinical response is unsatisfactory or steroid side effects are unacceptable (Table 2). These include dexamethasone, methylprednisolone, budesonide or ciclosporin23. Chlorambucil, considered as a chemotherapeutic agent, is becoming more popular as a second immunosuppressive drug in cats with IBD refractory to prednisolone alone. A complete blood count should be performed every three to four weeks to monitor for myelosuppression. The treatment approach for cats with small cellular lymphoma is the same as for severe IBD (that is, a combination of prednisolone and chlorambucil)22. Other chemotherapy protocols should be considered in large cellular (mostly B cell) lymphomas. Cobalamin is now well established as a complementary treatment for cats with IBD or lymphoma. In fact, many cats with chronic GI signs receive cobalamin supplementation regardless of their endogenous levels, so it is often left unmeasured23.
The normal or optimal intestinal microbiome composition in healthy cats is unknown, even though massive advances in the understanding of its composition have been made in recent years. The microbiota vary with age, diet, body condition and environmental factors25.
Remarkable variation can be found among healthy cats, and what is optimal for one cat, may be suboptimal for another25. Despite this, five main bacterial phyla tend to predominate: Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria25,26. Within the dominant Firmicutes phylum, members of the Clostridia class and Clostridiales order usually account for the highest percentage of sequences in feline faecal samples26. This is noteworthy because of the widespread notion clostridia are pathogens25.
When comparing microbiota from healthy and diseased animals, one major challenge is to differentiate cause and effect. However, as alterations in the gut microbiota seem to be consistently associated with diseases, such as IBD, measures to stabilise or restore its composition seem appealing25. This can be achieved indirectly – for example, by diet change or supplementation with prebiotics/dietary fibre – or directly by the administration of antimicrobials or probiotics (allegedly beneficial microorganisms).
Even though some evidence shows dietary modification – as well as the addition of fructooligosaccharides, cellulose, or pectin – can change the microbiota in healthy cats, extrapolating these data to clinical use is challenging, as the clinical relevance of these changes is still unknown25.
Source: Webb and Webb23
Probiotic therapy is common and has received much attention in human medicine with mixed results25. Convincing feline data for the efficacy of probiotics are lacking. No change in the microbiota was noted in a study of healthy cats – though subtle, yet relevant, changes may be difficult to identify27. In addition, it is unclear which type of probiotic is best suited for which host species, or indeed if probiotics have to be species-specific.
Evidence exists for variability in the response, or lack of response, with different probiotic strains in different hosts and different conditions25. Two studies in cats found benefit in the administration of a single organism to shelter cats with acute diarrhoea, or the use of a multi-organism product in client-owned cats with chronic enteropathy, respectively28,29.
Panel 1 lists feline GI conditions where probiotic supplementation may potentially be beneficial. These are, however, loosely based on theory, extrapolation from the human model and anecdotal reports or experience23. One of the more recent findings is the positive effect on T foetus diarrhoea relapse30.
In conclusion, much more needs to be learned about the best probiotic-host combination in specific diseases to make more evidence-based treatment recommendations.