19 Aug 2025
Equine allergic skin disease: triggers and treatment options
Emily Floyd BVSc, CertAVP(VD), DACVIM, MRCVS, discusses the diagnosis, medications, prevention and ongoing management for a common concern in horses

Figure 1. Pony with atopic dermatitis demonstrating a positive itch response to scratching the withers area.
Allergic skin disease is common in horses and has a significant negative impact on equine health. Allergic skin disease results from an exaggerated immune response to normally harmless allergens. In predisposed individuals, allergens are processed by antigen-presenting cells (such as Langerhans cells in the skin) and presented to T-helper (Th2) cells.
Th2 cells produce cytokines (IL-4, IL-5, IL-13) and promote a B-cell isotype switch with the production of IgE antibodies specific to the allergen. IgE binds to mast cells to prime them. When the horse is re-exposed to the allergen, it binds to IgE on mast cells, which triggers degranulation and the release of histamine, proteases, prostaglandins and leukotrienes.
This results in vasodilation, increased vascular permeability, oedema, inflammation in the skin and pruritus. Multiple risk factors influence the development of allergy, including environmental conditions, levels of allergen exposure, age at exposure and genetics.
Clinical approach to horse with suspected allergic skin disease
When dealing with a horse with suspected allergic skin disease, it is important to consider and exclude non-allergic causes of pruritus first. Common causes of pruritus are shown in Table 1.
History
A thorough history is essential and will often give a major indication as to likelihood of a diagnosis of allergic dermatitis and possible triggers. It should start with signalment and include a full review of onset, progression and duration of signs.
Seasonality is really important; did signs occur or worsen in the insect-biting season, or when pollen counts were high? Allergies typically progress with age, so in many cases pruritus can initially be seasonal, before becoming a continual problem.
Accurate information should be obtained about the horse’s environment, including bedding, type of stabling, ventilation, field location, proximity to hedgerows, crops and standing water. Any changes in environment should be noted. Diet review is important as, although rare, cutaneous adverse food reaction (CAFR) can occur. This should include consideration of forage, pasture, concentrate feeds and supplements. Full details of any treatments trialled and responses should be obtained. This should include all topical products, as contact allergy to products can exacerbate symptoms.
Fly control measures, including use of insect repellents or fly rugs, should be considered. Specific questions should be asked about the involvement of other horses or people, and whether any other genetically related horses are known to have symptoms. It is useful to ask the client for a pruritus score (0 to 10) to assess the severity of the problem.
Physical examination
A thorough general and dermatologic examination is essential. This is aimed at identifying clinical signs likely to be associated with allergy/pruritus, but also identifying non-allergic causes of pruritus, which may be the primary problem or confusing the clinical picture.
Crude assessment of the level of pruritus can be obtained by assessing response to scratching the withers area (Figure 1) and observing the horse in a relaxed state (Figure 2).

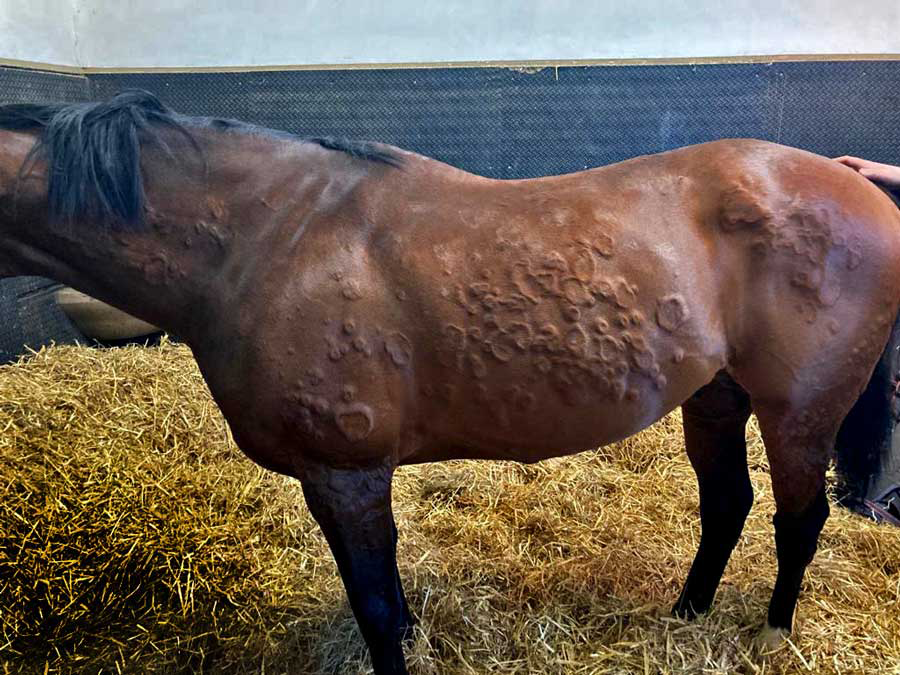
Typical allergic lesions include traumatic alopecia, erythema, papules and urticaria (Figure 3). Secondary infection can complicate allergy and is usually identified as raised tufts of hair (folliculitis), erythema, pustules and areas of alopecia, sometimes with changes in skin pigmentation.
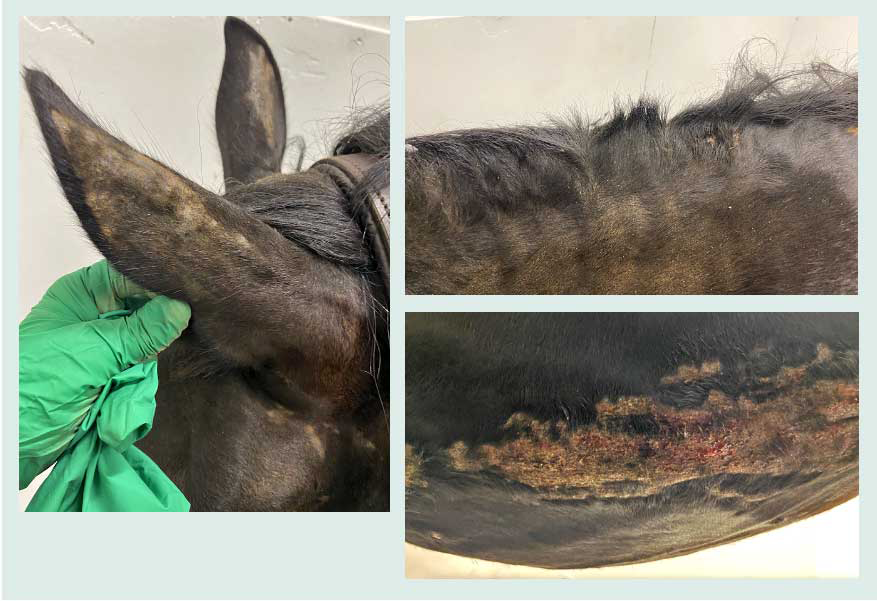
Close attention should be paid to identify ectoparasites. Common sites for this are lice under the mane area, Chorioptes bovis infestation of the lower limbs, and Oxyuris equi infection leading to perianal pruritus. Lesion distribution is also helpful; for example, lesions around the face, ears and ventral midline are commonly indicative of insect bite hypersensitivity (IBH; Figure 4).
Basic diagnostic tests
Basic diagnostic tests should be performed when any evidence presents of secondary infection, or concern exists about ectoparasites. Simple adhesive tape strips can be taken from the surface of lesions, stained using Diff-Quik stain and examined under the microscope for evidence of inflammatory cells, bacteria, Malassezia species, ectoparasites or fungal infection. Samples should be collected for culture from any horse with signs of bacterial infection that has a history of prior antimicrobial treatment.
Feather brushings (or adhesive tape strips) from the lower limbs should be carried out in feathered breeds with signs of lower-limb pruritus to check for the presence of Chorioptes bovis infestation.
Skin biopsies are rarely useful, and usually show signs of diffuse perivascular dermatitis. They can be useful to rule out other diseases, such as pemphigus foliaceus, although this is not usually pruritic in the horse. General haematology and biochemistry can be useful if any concerns exist about underlying systemic disease.
Specific diagnostic tests
Atopic dermatitis and IBH are clinical diagnoses made by careful assessment of the horse, environment and history, and exclusion of other pruritic diseases.
Allergy testing should never be used to make a diagnosis, as IgE antibodies to many allergens are present in non-allergic horses. The purpose of allergy testing is to identify triggers to aid in clinical management and when immunotherapy is being considered.
Options for allergy testing include intradermal testing or serologic testing. Both methods have their limitations, and correlation can be poor between the different methods.
Generally, intradermal testing is considered the best diagnostic option (Figure 5). Small amounts of specific allergens are injected into the skin. IgE antibodies in the skin bind to mast cells, leading to degranulation, release of histamine and other inflammatory mediators producing a wheal. Advantages include immediate results, ability to select a panel of relevant allergens to test, and assessment of IgE response directly in the skin.
Disadvantages include the need for withdrawal of steroid and antihistamine medication two weeks prior to testing, time taken to complete the test, compliance and false-negatives if allergen degradation occurs prior to testing (particularly a problem with Culicoides antigens).

Serologic tests have the advantage that sampling is easier and a wider panel of allergens can be run. Withdrawal of medications prior to testing is also not required. The major disadvantage is the possibility of false-negative results due to a lack of circulating IgE antibodies.
Test selection is absolutely essential. For the best results, the author recommends choosing a test that uses a recombinant high-affinity IgE receptor rather than those that use a standard IgE ELISA. Newer molecular testing is also now available. A combination of intradermal and serologic testing can be used if finances are not restricted.
If CAFR is suspected then a diet trial should be carried out. This can be done in different ways, but generally the easiest way is to feed a diet comprised of a single source of grass hay, supplemented with grass pellets if required. All other concentrate feeds, licks and supplements should be removed. This diet should be fed for a minimum of four to six weeks. If symptoms improve then CAFR may be suspected. To confirm the diagnosis, the previous diet should be reintroduced, one feed at a time, to observe for a recurrence of symptoms.
Treatment of allergic skin disease
Management relies on a multifactorial approach and the institution of multiple small interventions, which cumulatively push the level of pruritus below the allergic threshold.
Frustration and failure in management can occur when clients are not adequately counselled about the need for a multifaceted approach. It is also important that clients understand that allergic skin disease is rarely cured, but instead requires lifelong management.
Treatment can be divided into different areas:
- reducing allergen exposure
- reducing inflammation in the skin
- improving skin barrier function
- controlling any secondary infections
- modulating the immune response
Environmental management and reducing allergen exposure
When allergy testing has been performed, it is easier to put specific environmental changes into practice.
However, as allergen exposure is cumulative, it is generally beneficial to try to reduce exposure to all common allergens in all allergic horses.
Insect avoidance is the most important management tool in horses with IBH. Important recommendations include stabling at dawn and dusk, which are the peak feeding times for Culicoides species, keeping horses away from standing water or hedgerows, use of stable fans to disrupt insect flying and use of insect proof rugs. Use of effective fly repellents is essential. The most effective products contain a minimum of one per cent permethrin and must be applied daily.
Products that claim to have long-acting properties are unlikely to last for the reported time period and should be applied more frequently. Some essential oil products may have some repellent effect, but are not as effective as permethrin-based products.
Many atopic horses are allergic to dust and storage mites, so control of allergen exposure in the indoor environment is essential. Full pasture turnout is the ideal intervention for dust-mite allergic horses. If this is not possible then small amounts of low-dust bedding that is removed daily should be used.
Stables should be cleaned and pressure washed regularly, hay and bedding stores should be kept away from the stable area and feed should be stored carefully to prevent storage mite infestation.
Vacuum-packed forages can have lower concentrations of dust mites than unwrapped forages. Rugs are a major source of dust mites and should be hot washed regularly.
Avoidance of tree, weed and pollen allergens can be more challenging. It can be useful to avoid turnout adjacent to trees/hedgerows or specific crops. The use of selective weed-killers on pasture can reduce concentrations of weed pollens, and in some cases minimising turnout at high pollen times is necessary.
Medications to control pruritus and reduce skin inflammation
For horses with severe pruritus, glucocorticoids remain the most effective and readily available medication. Doses are listed in Table 2. Dexamethasone is more potent, but prednisolone is generally considered safer for long-term maintenance use.
The lowest effective dose should be identified. Using every other day dosing will reduce side effects for the horse. Steroid-induced laminitis always remains a concern. Measurement of a resting insulin concentration in metabolically questionable individuals can be useful to help quantify the actual risk level. Steroids should be used cautiously if resting insulin concentration is high.
Antihistamines are rarely adequate for sole control of allergy, but can be useful as an adjunctive therapy, and can reduce the steroid dose required. Individual response to antihistamines is variable, but cetirizine is generally the first-line choice of medications currently available in the UK.
Oclacitinib is a Janus kinase inhibitor that is widely used in small animals and may have a role in horses. Cost is a major limitation to use, though.
Omega-3 supplementation/ skin barrier function
Omega-3 can be a useful adjunctive therapy to improve skin barrier function and reduce skin inflammation. Fish or algal sources are better incorporated into the skin than plant-based supplements.
Control of secondary infections
Topical therapy is preferable, using medicated shampoos containing chlorhexidine-based products. Other useful products include chlorhexidine mousse and hypochlorous acid sprays.
Systemic antimicrobials may be needed in some cases, but when chosen should be used for an adequate duration, and ideally based on results of culture and sensitivity. Bathing also has the advantage that it removes surface allergens from the coat.
Allergen-specific immunotherapy
Subcutaneous injections of immunotherapy created specifically for an individual horse based on identified allergens can help modulate the immune response over time.
Allergen-specific immunotherapy contains environmental allergens identified by serologic or intradermal testing. Immunotherapy for Culicoides species is not effective, but this may change as further work is being carried out to identify the target antigens. Treatment should ideally be lifelong, but for a minimum of two to three years. Improvements are expected in 60 per cent to 70 per cent of horses.
In the future, cytokine-based vaccines may have a role in modulating the Th2 response.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
- This article appeared in Vet Times Equine (BEVA Congress Autumn 2025), Volume 11, Issue 3, Pages 5-7, and previewed the author’s session at the congress.
- Non-responsive pruritus cases: guide to potential options – another equine dermatology article from the Vet Times archive.
Author
Emily Floyd graduated from the University of Bristol in 2003. She completed a residency in large animal internal medicine at UC Davis and became a diplomate of the American College of Large Animal Internal Medicine. Emily works as a clinical director at Rossdales Equine Hospital in Newmarket. She enjoys all areas of internal medicine, but has special interests in neonatology and dermatology.
References
- Hunyadi L, Datta P, Rewers-Felkins K, Sundman E, Hale T, Fajt V and Wagner S (2022). Pharmacokinetics of a single dose of oclacitinib maleate as a top dress in adult horses, Journal of Veterinary Pharmacology and Therapeutics 45(3): 320-324.
- Loeffler A, Herrick D, Allen S and Littlewood JD (2018). Long-term management of horses with atopic dermatitis in southeastern England: a retrospective questionnaire study of owners’ perceptions, Veterinary Dermatology 29(6): 526-e176.
- Marsella R, White S, Fadok VA, Wilson D, Mueller R, Outerbridge C and Rosenkrantz W (2023). Equine allergic skin diseases: Clinical consensus guidelines of the World Association for Veterinary Dermatology, Veterinary Dermatology 34(3): 175-208.
- Marsella R (2024). Pruritic horse: approach to allergic skin diseases in horses, Veterinary Clinics of North America: Equine Practice 40(2): 219-235.
- Morgan EE, Miller WH Jr and Wagner B (2007). A comparison of intradermal testing and detection of allergen-specific immunoglobulin E in serum by enzyme-linked immunosorbent assay in horses affected with skin hypersensitivity, Veterinary Immunology and Immunopathology 120(3-4): 160-167.
- Stepnik CT, Outerbridge CA, White SD and Kass PH (2012). Equine atopic skin disease and response to allergen-specific immunotherapy: a retrospective study at the University of California-Davis (1991-2008), Veterinary Dermatology 23(1): 29–e7.
- White SD (2023). Approach to the pruritic horse, Journal of the American Veterinary Medical Association 261(S1): S66-S74.
