22 Jul 2025
Nicola Gladden BVM&S, DipECBHM, PhD, PGCHE AFHEA, MRCVS reviews a condition recognised for more than a century and a half, including how it can be diagnosed, prevented and treated
Image: sabbra_cadabra / Adobe Stock
Ketosis is an important disorder of dairy cows that has been recognised for more than 150 years (Mann and McArt, 2023; Rico and Barrientos-Blanco, 2024).
Ketogenesis is a healthy physiological response when glucose supply is insufficient to meet demand. However, if cows enter negative energy balance (NEB), circulating ketone quantities can become excessive and pathological. Increased serum ketone concentration (hyperketonaemia) is most commonly diagnosed in the first three weeks postpartum, and at this point in lactation is related to excessive mobilisation of adipose tissue around calving.
At peak lactation, hyperketonaemia may be diagnosed in high-producing cows whose lactational energy demands exceed supply. Historically, these two presentations were classified as “type II” and “type I” ketosis respectively, based on hypothesised similarities to diabetes mellitus (Holtenius and Holtenius, 1996; Table 1), but recently this hypothesis has been challenged (de Koster et al, 2016; Mann et al, 2016; Zachut et al, 2013).
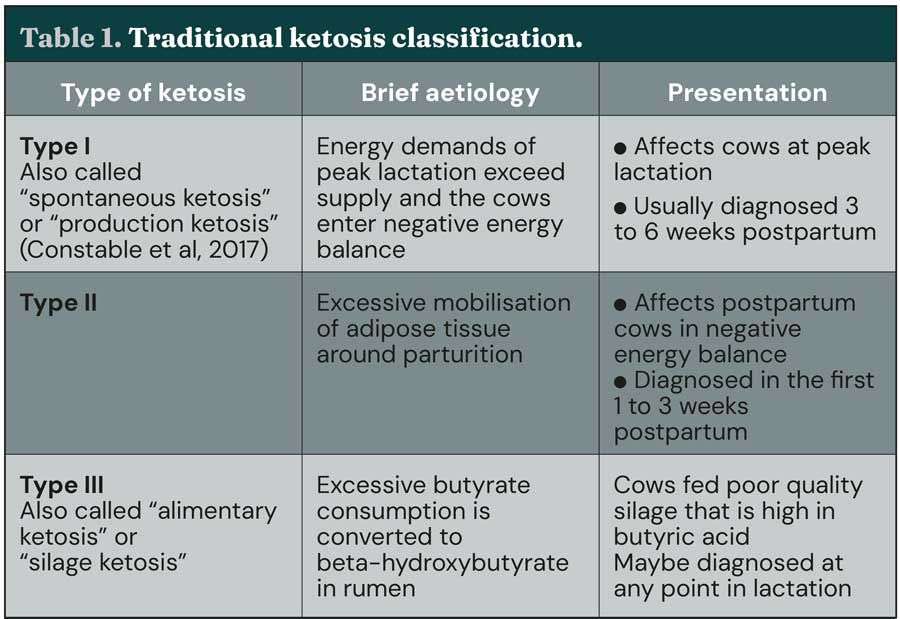
As such, although the terms type I and type II ketosis are still used (for example, Zhang and Ametaj, 2020), it has been suggested that this classification method should be retired (Mann and McArt, 2023). A third (less common) type of ketosis has also been described and occurs when cows consume poor-quality silage high in butyric acid that is converted to beta-hydroxybutyrate (BHB) in the rumen.
Ketosis can also be defined as “subclinical” or “clinical” based on defined diagnostic thresholds of greater than or equal to 1.2mmol/L and greater than or equal to 3mmol/L, respectively (Table 2). However, the value of describing ketosis in this way is currently subject to debate, as it is now known that not all cows with high circulating ketone concentration present with clinical signs.
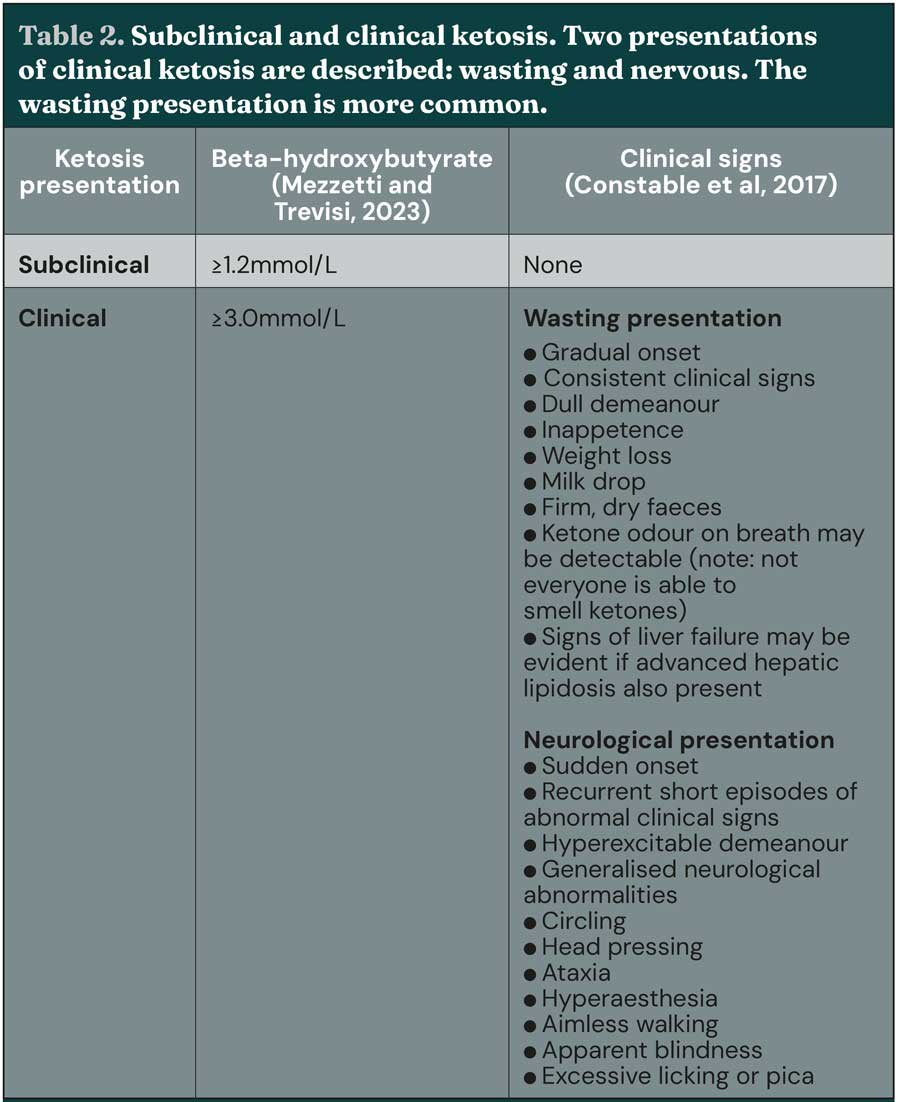
Additionally, it has been argued that as clinical and subclinical ketosis are both part of the same syndrome, differentiation has little clinical relevance (Mann and McArt, 2023). In the past decade, the term hyperketonaemia has increased in use, as it more accurately reflects the observation that an elevated concentration of circulating ketone bodies does not in itself represent a disease process, whereas ketosis is a recognised disease.
It is known that increasing severity of hyperketonaemia indicates a more severe energy deficit and to reflect this it has been suggested hyperketonaemia is reclassified as moderate (BHB of 1.2mmol/L to 2.9mmol/L) or severe (BHB more than or equal to 3mmol/L; Caixeta and Omontese, 2021).
Hyperketonaemia is associated with adverse health events, premature culling and reduced fertility (Abdelli et al, 2017; Duffield et al, 2009; McArt et al, 2012, 2013a; Raboisson et al, 2014). Some studies have also reported reduced milk production in cows affected by hyperketonaemia (for example, Duffield et al, 2009), but the available data are inconsistent with other studies finding that milk yield of hyperketonaemic cows is higher than healthy cows (for example, Ruoff et al, 2017). These conflicting findings may be related to study design, as illustrated by Mann and McArt (2023) who reviewed currently available data accounting for the timing of ketone testing relative to calving.
Analysing the literature in this way highlighted that negative effects of hyperketonaemia on milk yield are more pronounced in studies that sampled cows in the first week of lactation compared to studies that measured ketone concentration after seven days in milk (DIM). This finding is supported by other studies that found hyperketonaemia diagnosed in the first week of lactation is associated with poorer health and productivity outcomes compared to if hyperketonaemia is diagnosed later in lactation (McArt et al, 2012; Rodriguez et al, 2022). Therefore, timing of diagnosis may be an important consideration when interpreting ketone test results, and some researchers have hypothesised that serum ketone concentration of more than or equal to 1.2 mmol/L after 7 DIM may represent a healthy adaptation to the demands of lactation in high-yielding dairy cows, rather than a pathological process (Mann and McArt, 2023).
Hyperketonaemia has significant economic impacts (Cainzos et al, 2022). European studies that have modelled the economic costs associated with hyperketonaemia estimate the cost of a single case to be €150 to €257 (£126 to £215 at time of writing), with premature culling being the biggest contributor (Raboisson et al, 2015; Steeneveld et al, 2020).
By contrast, an American study found that although the average estimated overall cost per case was similar to European studies (US$289, equivalent to £214 at time of writing), reduced reproductive performance was the biggest contributor (McArt et al, 2015). Additionally, it is increasingly being recognised that reduced productivity resulting from disease is likely to affect the environmental sustainability of cattle farming (Capper and Williams, 2023; Džermeikaitė et al, 2024).
Few studies evaluate the environmental impacts of ketosis, but one study found that hyperketonaemia is associated with increased greenhouse gas emissions per tonne of milk – a result principally related to reduced production efficiencies and increased culling (Mostert et al, 2018).
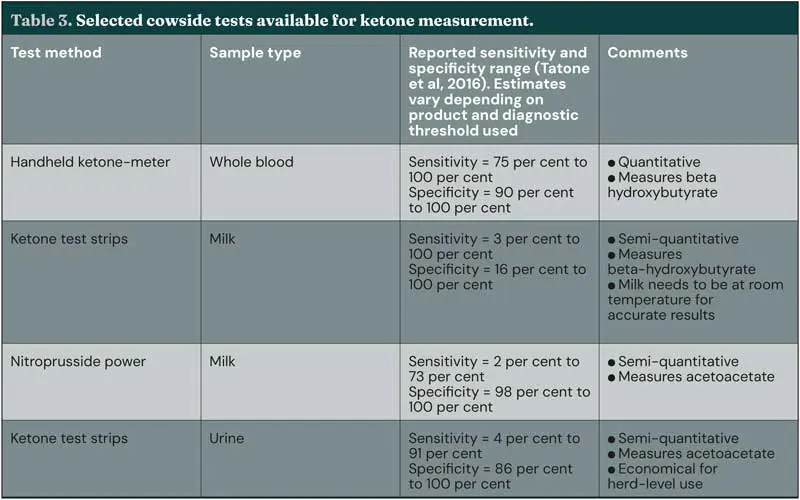
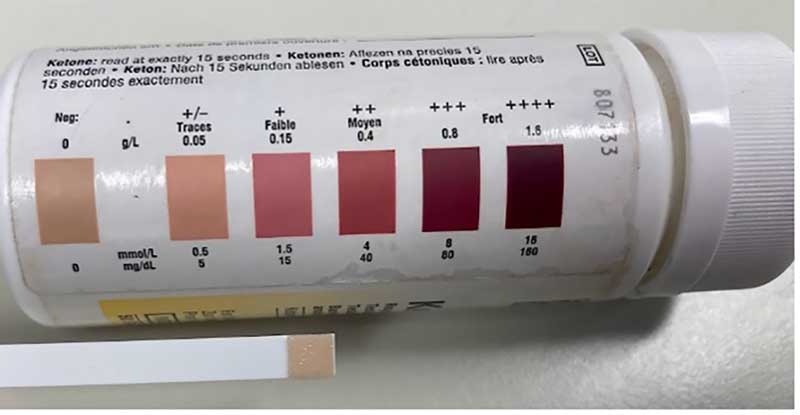
Hyperketonaemia can be diagnosed by testing blood, milk, or urine; several validated test methods are available (Table 3; Tatone, Gordon et al, 2016). Clinical ketosis is diagnosed by evaluation of risk factors (Table 4) and clinical presentation (Table 2), together with circulating ketone concentration. Measurement of BHB in whole blood using a handheld ketone meter is considered optimal, particularly for diagnosis of individual cows (Mann and McArt, 2023; Tatone, Gordon et al, 2016) – a diagnostic threshold of greater or equal to 1.2mmol/L has optimal sensitivity and specificity (Tatone, Gordon et al, 2016).
Urine testing (Figure 1) offers an economical alternative for routine testing of large numbers of animals to collect herd-level data, but, while urine testing is suitable for herd-level screening (Carrier et al, 2004), the reduced accuracy of urine tests compared to blood tests means measurement of urine ketone concentration is unsuitable for individual diagnosis. Additionally, research work has found cowside urine and milk tests both detected ketotic cows two days later than a cowside blood test (Serrenho et al, 2022) – factors for consideration when using these tests in practice.
Monitoring how well dairy cows transition into lactation is crucial for early identification of potential problems. Detailed discussion of transition monitoring strategies is beyond the scope of this article, but housing and environment, body condition score (BCS), postpartum cow health, early lactation milk production and feed management should all be evaluated (Caixeta and Omontese, 2021). Routine measurement of indicators of energy balance has a valuable part to play in these wider herd monitoring strategies.
Measurement of circulating ketones, as previously discussed, can be performed routinely for herd-level monitoring. While sampling all postpartum cows is optimal, this may not be feasible – alternative approaches include targeted testing of high-risk cows or regular testing of a subset of at-risk cows (Leblanc, 2010; Mann and McArt, 2023). Selection of an appropriate sample size depends on the number of animals at risk, the expected hyperketonaemia prevalence and the confidence and precision required (Ospina et al, 2013), but sample sizes of 10 to 20 are typically suggested (Leblanc, 2010; Mann and McArt, 2023; Mezzetti and Trevisi, 2023).
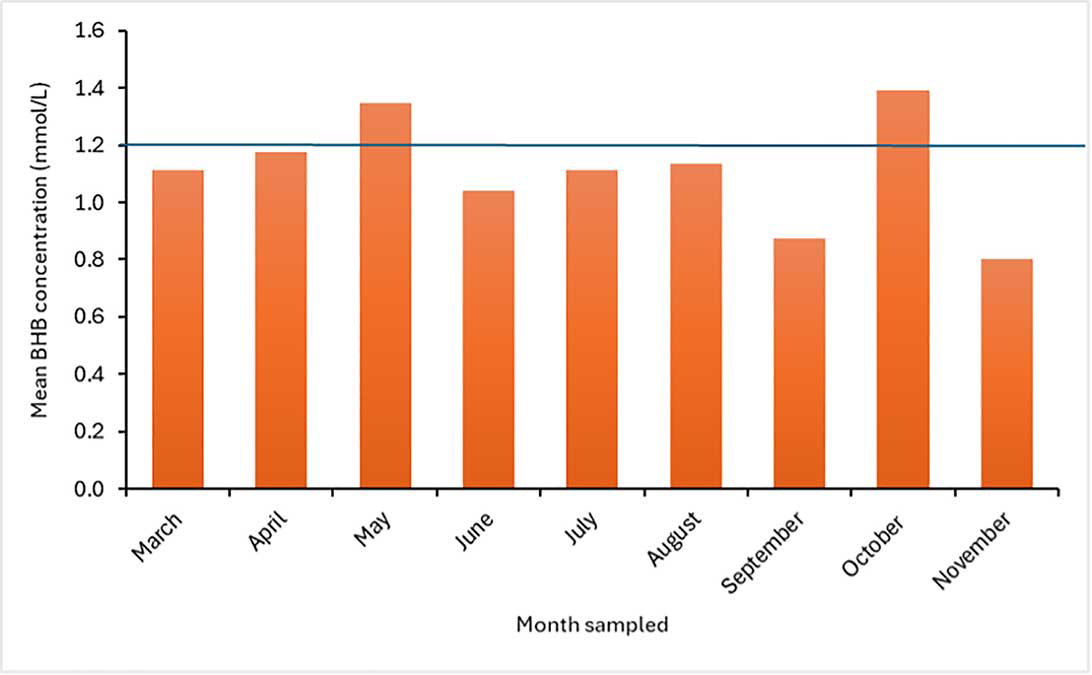
For herd level analysis, monitoring the proportion of sampled cows with BHB concentration greater or equal to 1.2mmol/L is useful (Figure 2); it is suggested to aim for a prevalence of less than 15 per cent and to set an alarm threshold of more than or equal to 25 per cent (Caixeta and Omontese, 2021). Calculating mean BHB concentration of a cohort of tested animals is not recommended, as results can be misleading (see example in Figure 3).
![Figure 2. Beta-hydroxybutyrate (BHB) data routinely collected from cows 1 to 14 days in milk. Graph shows the prevalence of hyperketonaemia (BHB ≥1.2mmol/L) and highlights this farm has energy balance issues in fresh cows, with the monthly hyperketonaemia prevalence exceeding the target of 15 per cent (lower horizontal [orange] line) and the intervention threshold of 25 per cent (upper horizontal [red] line) in most months.](https://testing-vettimes-next.sfo3.digitaloceanspaces.com/2025/09/Gladden-Fig-2.webp)
Prepartum serum NEFA concentration is a better predictor of postpartum disease than BHB concentration, but measurement of BHB is less expensive and can easily be performed on farm. Accordingly, for clinical practice BHB is recommended as the first line testing method for routine herd monitoring. Measurement of NEFAs is advised for herds that are having problems, or herds where routine BHB measurements are within healthy limits, but NEB is still suspected (Ospina et al, 2013).
Hyperketonaemia is associated with an increase in milk fat concentration and a decrease in milk protein concentration, meaning the fat to protein ratio (FPR) of milk can be used as an indirect measure of energy balance (Cabezas-Garcia et al, 2021). An FPR of 1.0 to 1.5 is considered to be normal, with an FPR greater than 1.5 classed as high (indicative of NEB).
An FPR greater than 2.0 in the first 10 DIM has been associated with an increased risk of postpartum disease and culling (Toni et al, 2011), but FPR has consistently been found to perform poorly as a measure for detecting individual cows with hyperketonaemia (Cabezas-Garcia et al, 2021; Duffield et al, 1997; Løvendahl et al, 2010). As such, FPR is not advised to be used for diagnosis of hyperketonaemia in individual cows. However, FPR can be a useful tool to monitor herd-level energy balance and for herd screening to determine which cows would be most suitable for blood testing (Jenkins et al, 2015). Fourier-transform infrared (FTIR) spectrometry is already routinely used to analyse milk quality (McParland and Berry, 2016) and may have potential for detection of NEB.
To date, studies have found that FTIR shows promise for prediction of cows at high risk of developing postpartum disease (Bach et al, 2019; Cascone et al, 2022). However, diagnosis of hyperketonaemia at individual level is reportedly suboptimal (Caldeira et al, 2020), so while this test may become a useful tool in the future, further work is needed before routine use can be implemented.
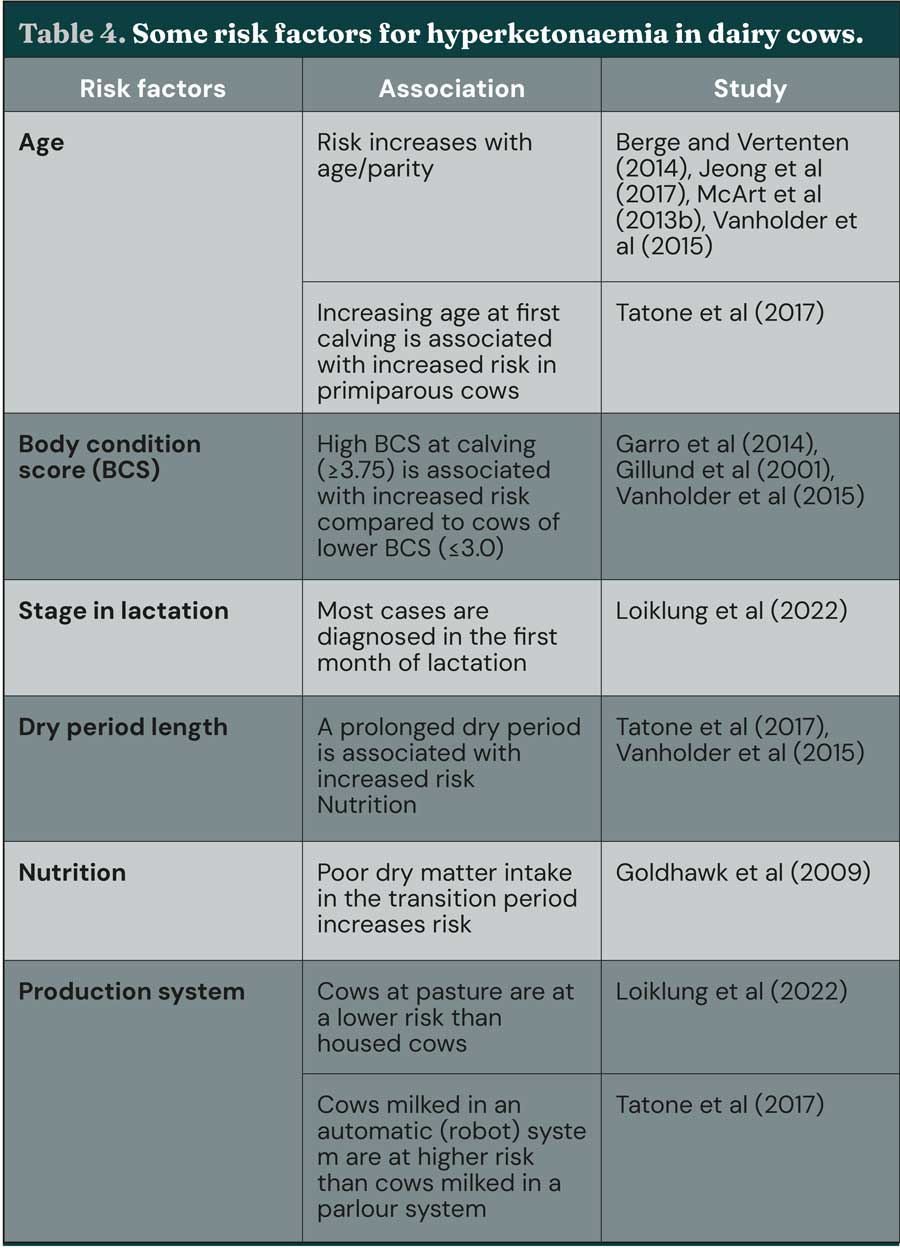
Management of risk factors (Table 4) is key to preventing ketosis: ensuring adequate dry matter intake in the transition period, optimising BCS in late lactation, avoiding excessive BCS loss around calving and maximising feed intake after calving are all crucial Transition management has been well-reviewed elsewhere (for example, Atkinson, 2016; Cardoso et al, 2020; Overton and Waldron, 2004; van Saun and Sniffen, 2014), and readers are encouraged to refer to these texts for further detail. Inclusion of gluconeogenic precursors in the diet may also be of benefit on some farms – these are reviewed elsewhere (Melendez and Serrano, 2024).
Treatment of ketosis should aim to correct the underlying metabolic derangements, rather than be solely focused on reduction of ketone concentration. Few studies are available to support common treatment approaches for ketosis (Gordon et al, 2013; Mann and McArt, 2023), but the strongest evidence basis is for treatment with oral propylene glycol (PG), with studies consistently reporting improvements in health and performance outcomes in treated animals (Cascone et al, 2022; Gordon et al, 2013; McArt et al, 2011). PG treatment of animals presenting with hyperketonaemia in the absence of clinical signs (namely, subclinical ketosis) is also beneficial, as it reduces the risk of developing other diseases and has been associated with improved milk yield (Cascone et al, 2022; McArt et al, 2011).
The standard recommendation is to administer 300g propylene glycol orally once daily for three days to cows presenting with serum BHB concentration greater to or equal to 1.2mmol/L; extending this to a five-day course is beneficial for cows presenting with higher BHB concentration (greater than 2.4mmol/L; Gordon, LeBlanc, et al, 2017).
It is notable that one study found blood BHB concentration only decreased for a few hours in response to PG treatment (Mann et al, 2017), so care should be taken when monitoring BHB concentration to assess treatment response, as this may be misleading. Clinical assessment of the health and milking performance of affected cows may be a more accurate indicator of treatment response, but Mann et al (2017) did not include clinical parameters in their study, so the clinical importance of only a transient decrease in blood BHB concentration following PG treatment is unclear.
Reports have been made of PG toxicity in cattle, but the bovine toxic dose threshold is uncertain, with one review finding that reported toxic doses range from 150g to 1,500g for adult cows (Nielsen and Ingvartsen, 2004). Although toxic effects are unlikely to be observed when treating cows with the standard dose of 300g/day, it is still worth advising farmers of abnormal clinical signs to look for that may indicate toxicity, such as ataxia, depression, hypersalivation or sulphuric-smelling breath.
Glucocorticoids have been used in the treatment of ketosis for a long time due to their well-established gluconeogenic effect in monogastric species. However, it is less clear if this occurs in the same way in ruminants. Few data are available to support the use of glucocorticoids for treatment of ketosis. Moreover, cows treated with glucocorticoids in addition to PG have been found to have a poorer response to treatment and reduced subsequent reproductive performance compared to cows treated with PG alone (Tatone, Duffield et al, 2016). As such, glucocorticoid treatment is no longer recommended.
Dextrose has also historically been used to treat ketosis, but there is little evidence to support routine use. Although serum BHB concentration has been shown to reduce in response to IV dextrose administration (500ml, 50 per cent solution), this effect lasts less than four hours (Mann et al, 2017).
Treatment with IV dextrose and oral PG (300ml once daily for three days) has been found to have a more prolonged effect than treatment with either dextrose or PG alone (Mann et al, 2017), so dextrose may be useful as an adjunct treatment for ketosis.
Available data are limited regarding the effects of dextrose treatment on longer-term outcomes (for example, health or productivity), but one study found that treating hyperketonaemic cows with IV dextrose and oral PG treatment for three days did not benefit milk yield or subsequent health events any more than treatment with PG alone (Capel et al, 2021); therefore, dextrose treatment is not currently recommended for routine treatment of hyperketonaemic cows. Nevertheless, dextrose administration may be of benefit in selected cases where a rapid decrease in serum BHB concentration is needed – for example, nervous ketosis.
Other treatment approaches that have been studied include vitamin B12, glycerol, and amino acid supplements.
Improvements in resolution of hyperketonaemia and milk yield have been reported when vitamin B12 or amino acids are administered in addition to PG, and these products may have value as adjunct therapies, but to date, the benefits of administering these products alone is unknown (Gordon, Duffield, et al, 2017; Gordon, LeBlanc, et al, 2017; Jeong et al, 2018). Glycerol is a glucose precursor that targets a different point in the glucose pathway to PG, and as such may have potential for treatment of ketosis.
A small study of two cows diagnosed with clinical ketosis found that hyperketonuria responded to glycerol treatment in both cows (Goff and Horst, 2003). This was a very small study, though, and the results have been challenged by later work that found PG was a more effective ketosis treatment than glycerol (Piantoni and Allen, 2015). These results are supported by a study of pregnant sheep that found that while serum glucose concentration increased rapidly following glycerol treatment, serum BHB and NEFA concentrations responded better to PG (Alon et al, 2020).
Hyperketonaemia and ketosis are commonly diagnosed in dairy cattle and often have adverse effects on health and productivity. Diagnosis of individual cows is straightforward to perform on farm, and herd-level monitoring is a useful tool for detecting problems and enabling early targeted intervention with control measures.
Research in the past decade has expanded our understanding of this disorder but evidence-based routine treatment options remain limited to oral administration of propylene glycol and further studies are warranted to identify alternative therapeutic approaches, especially for cows that are refractory to standard treatments.
Nicola Gladden qualified from The University of Edinburgh in 2006 and worked in mixed clinical practice before taking up a position as senior clinical scholar in production animal health at the University of Glasgow School of Veterinary Medicine in 2014. Nicola qualified as a diplomate of the European College of Bovine Health Management in 2019 and completed a PhD in 2021. She is a European and RCVS specialist in bovine health and production and works at the University of Nottingham School of Veterinary Medicine and Science teaching farm animal practice to undergraduate veterinary students, as well as continuing to do some clinical work.