12 Aug 2025
Dermatophytosis in dogs and cats – a comprehensive review
Davide Immediato DVM, PhD, GPCert(Derm), MRCVS Filippo De Bellis DVM, CertVD, DipECVD, MRCVS offer an overview of ringworm in pets, including aetiology, treatment strategies and preventive measures.

Image: Nynke / Adobe Stock
Dermatophytosis, commonly known as ringworm, represents a superficial fungal infection affecting the skin, hair and nails of both canine and feline populations1-4. This condition is not merely a dermatological concern; its zoonotic nature (transmission between animals and humans) elevates its importance in the context of companion animal and public health1,3-5.
Managing dermatophytosis can be particularly challenging in environments housing multiple animals, such as shelters, due to its highly contagious nature and the associated risk of transmission to humans5,6.
This review article aims to provide an overview of dermatophytosis in dogs and cats, encompassing its aetiology, epidemiology, clinical manifestations, pathogenesis, diagnosis, current treatment strategies, preventive measures and zoonotic potential.
Aetiology of dermatophytosis
A group of closely related filamentous fungi known as dermatophytes, which possess the unique ability to colonise keratinised tissues (such as hair, skin nails, and feathers), causes dermatophytosis1,7.
These fungi belong to the phylum Ascomycota, class Eurotiomycetes, order Onygenales, and family Arthrodermataceae7. Seven accepted genera of dermatophytes exist: Trichophyton, Epidermophyton, Nannizzia, Paraphyton, Lophophyton, Microsporum and Arthroderma7. In the context of canine and feline infections, the genera Microsporum species and Trichophyton species are the most frequently implicated1-6,8. Among these, the most commonly isolated species include Microsporum canis, Microsporum gypseum and Trichophyton mentagrophytes (Table 1)1,3-6,8.
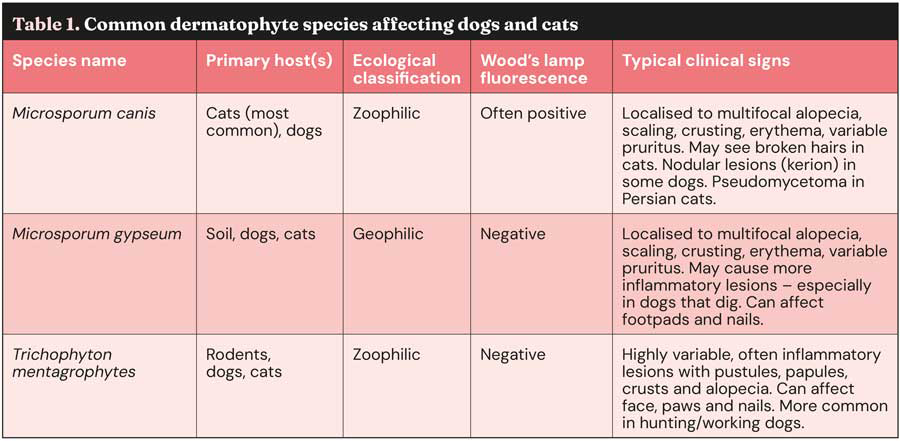
Dermatophytes can be further categorised based on their primary ecological niche as zoophilic species (adapted to animal hosts), geophilic species (reside in the soil) and anthropophilic species (primarily infect humans)3,5,9. M canis is predominantly a zoophilic dermatophyte and is most commonly transmitted to dogs and cats through contact with infected cats8. T mentagrophytes, another zoophilic species, is often associated with rodents and other wildlife, posing a risk to domestic animals that may come into contact with these reservoirs3,5,8.
M gypseum, in contrast, is a geophilic dermatophyte found in soil, and infection typically occurs through direct contact with contaminated soil3,5,6,8.
M canis is consistently identified as the most prevalent causative agent of dermatophytosis in dogs and cats3,5,8. The frequency of isolation of other species such as T mentagrophytes and M gypseum can vary depending on geographical location and the lifestyle of the affected animals – particularly their degree of exposure to outdoor environments and potential contact with wildlife or contaminated soil3,5,8.
Molecular approaches have been applied to dermatophytes to assist in classification and epidemiological studies7. Using two gene regions (internal transcribed spacer region and partial β-tubulin), Baert et al10 proposed a new classification scheme for dermatophyte species using a phylogenetic approach. This resulted in numerous species being reclassified, expanding the Nannizzia, Paraphyton, Lophophyton and Trichophyton genera while condensing the Arthroderma and Microsporum genera. This included renaming the clinically important species Nannizzia persicolor (former name Arthroderma persicolor), Nannizzia nana (former name Microsporum nanum), Trichophyton mentagrophytes (former name Arthroderma vanbreuseghemii), and Nannizzia gypsea (former name Microsporum gypseum)10,11.
Practitioners need to be aware of this, as clinical articles are increasingly using the new nomenclature4. However, this article will use the traditional names.
Epidemiology and transmission
Dermatophytosis exhibits a global distribution, affecting canine and feline populations worldwide1,3,7,12.
Epidemiological studies conducted across various regions have revealed a wide range of prevalence rates1. It is important to note that the true prevalence of dermatophytosis may be underestimated in many studies because it is not a reportable disease in pets, and many prevalence studies do not distinguish between animals with active infections and those that are simply carrying fungal spores on their coats (fomite carriage)3,4,13.
Dermatophytes are transmitted through several routes, including direct contact between healthy and infected animals; this is considered a primary mode of transmission4,5,7,8. Indirect contact via contaminated fomites (such as brushes, clippers, bedding, toys, and cages) is possible only if concurrent microtraumas exist4.
Environmental contamination via arthroconidia (infectious spores) has been reported as a rare event3,4. Arthroconidia can survive in the environment for extended periods from months to years, posing a risk of infection3,5,7,8.
Notably, many cats – particularly long-haired breeds – can be asymptomatic carriers of dermatophytes, meaning they harbour the fungus without showing any clinical signs, and contribute to the spread of infection3,4,5,8.
Pathogenesis, predisposing factors and host immune response
Dermatophyte infections begin with the adherence of arthroconidia to keratinised tissues. The arthroconidia then germinate and penetrate the stratum corneum, leading to hyphal growth and invasion of keratinised tissues1,3-5,7. To facilitate penetration into the keratinised tissue, dermatophytes produce various proteolytic and keratolytic enzymes3,7,14.
The initiation of infection requires several steps3,7,15.
- Adherence: arthroconidia adhere to the epidermis within two to six hours of contact. This adherence is facilitated by glycoproteins containing mannans in the cell wall of these fungi.
- Germination: the arthroconidia germinate and develop germ tubes that penetrate the stratum corneum.
- Invasion: fungal hyphae grow and invade keratinised tissues, utilising keratin as a nutrient source. The fungus also alters the local pH to become more basic, which aids in the activity of fungal proteases.
- Spread: within seven days of infection, hyphae begin producing arthroconidia, allowing the fungus to spread to other sites on the host or new hosts.
Several factors have been identified as increasing the risk of dermatophytosis in dogs and cats. Along with dermatophyte virulence factors, the host’s immune status is crucial1. Younger animals, such as puppies and kittens, are generally more susceptible to infection1,3,4,7,8. Certain breeds may also exhibit a predisposition to the disease; for instance, Yorkshire terriers and Persian cats have been reported to have a higher incidence in some studies3-5,7,8. Some research suggests a higher prevalence in female animals1. Animals with compromised immune systems, due to underlying health conditions or immunosuppressive therapies, are also at an elevated risk1,3-5,8.
Environmental factors, such as warm and humid climates, overcrowding, and poor hygiene, create conditions favourable for fungal growth and transmission1,3-5,7. Physiological stress can also play a role in increasing an animal’s susceptibility to dermatophyte infection1,3-5,8.
Finally, the lifestyle of the animal, such as working or hunting dogs with increased outdoor exposure, may elevate the risk3-5,13.
The host’s immune system mounts a response to combat dermatophyte infection. The innate immune response involves the action of neutrophils and macrophages, which are among the first immune cells to respond to the presence of the fungi14.
Fungal components, such as those found in the dermatophyte cell wall, are recognised by pattern recognition receptors such as toll-like receptors on immune cells1. This recognition triggers the production of pro-inflammatory cytokines, such as interleukin (IL)-1, IL-6, IL-10, IL-17, and tumour necrosis factor-α, which help to orchestrate the inflammatory response1. Cell-mediated immunity – particularly the T helper 1 cell response – is considered crucial for the effective clearance of dermatophyte infections1.
However, as mentioned previously, stress and elevated cortisol levels can interfere with the host’s immune response, potentially suppressing it and prolonging the infection1.
Although dermatophytes have this powerful metabolic machinery for survival, they still need to overcome many other pitfalls imposed by the host. Therefore, dermatophytes have developed mechanisms that allow them to avoid the host response, such as the immunosuppressive action of fungal mannans, which causes reduction of inflammation and phagocytosis, and the expression of the iC3b receptor, which competes with polymorphonuclear cells for C3b, limiting phagocytosis15.
Clinical signs and differential diagnoses
The clinical presentation of dermatophytosis in dogs and cats can be quite variable, depending on factors such as the specific dermatophyte species involved, the animal’s immune status, and the extent of the infection1.
However, several common clinical signs are frequently observed. Alopecia is a hallmark of dermatophyte infection, often appearing as localised patches that may sometimes exhibit a circular pattern, giving rise to the term “ringworm” (Figure 1)1,3-5,8.
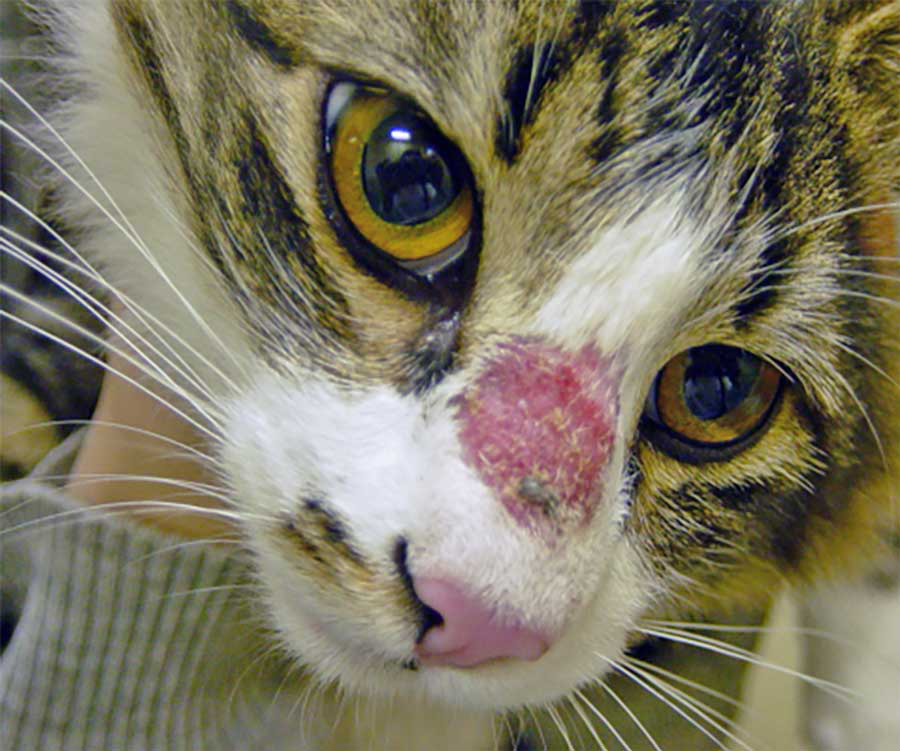
Scaling and crusting of the skin are also commonly seen in affected animals, often accompanied by skin erythema1,3-5,8. The degree of pruritus associated with dermatophytosis can vary significantly between individual animals1,3-5,8. In some cases, papules and pustules may also be present, and hyperpigmentation can occur in chronic infections1,3-5,8.
The clinical presentation can vary between dogs and cats. In dogs, lesions may be more frequently localised to the face, legs, and/or tail, whereas in cats, lesions may appear as irregular or circular areas of hair loss, with or without scaling1,3,5.
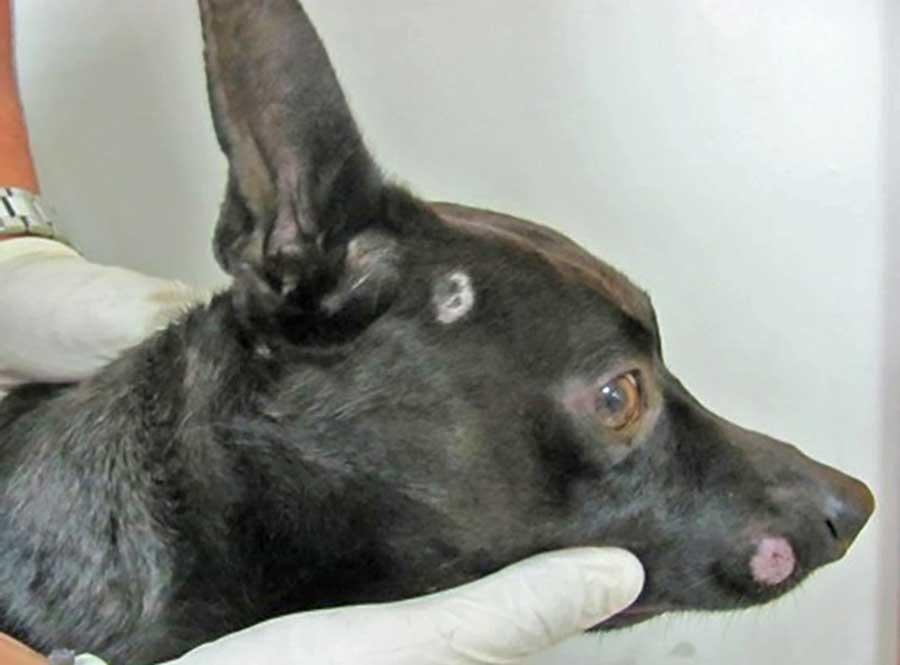
Certain breeds, such as the Persian cat and Yorkshire terrier, may be more prone to developing nodular lesions, including pseudomycetoma or mycetoma, and kerion, which are deeper, more inflammatory forms of the infection (Figure 2)3-5,8. Cats can also develop exudative paronychia, an inflammation of the tissue surrounding the nails4,8,13.
Given the range of clinical signs associated with dermatophytosis, it is essential to consider several differential diagnoses, including bacterial pyoderma, demodicosis, pemphigus foliaceus in presence of crusts and pustules, systemic lupoid onychodystrophy or genetically inherited dystrophies in cases involving nail lesions, pyotraumatic dermatitis in dogs, eosinophilic lesions in cats, and even neoplasia in cases where nodular lesions are present8,13,16. The variability in clinical signs and the common occurrence of asymptomatic carriers underscore the difficulty of diagnosing dermatophytosis based solely on clinical presentation. Therefore, the use of various diagnostic tools is often necessary to confirm the presence of an active fungal infection.
Diagnostic approaches for dermatophytosis
Diagnosing dermatophytosis involves a combination of several complementary diagnostic tests, as no single test has been identified as a definitive “gold standard” (Table 2)3,4,8.
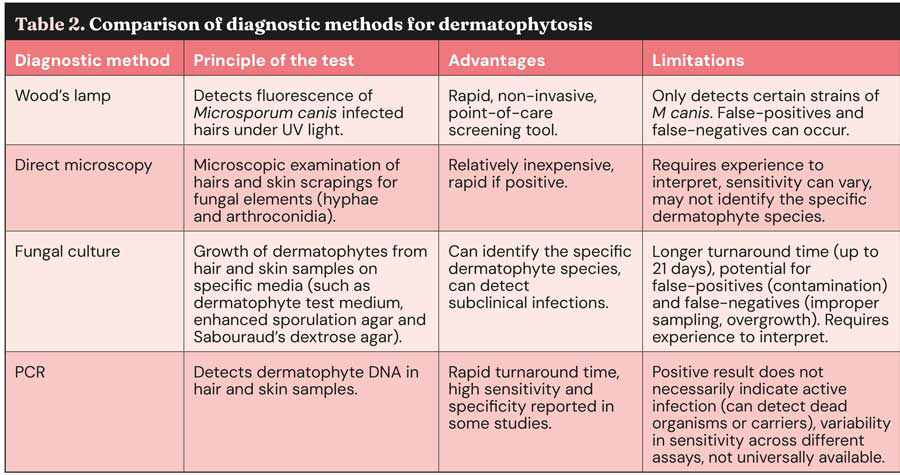
One commonly used initial screening tool is the Wood’s lamp examination, which utilises ultraviolet light (320nm to 400nm) to detect fluorescence in hairs infected with M canis, where a characteristic apple-green fluorescence is often observed (Figure 3)3-5,7,8,16,17. However, false-positive results can occur due to the presence of skin scales, sebum, certain topical products or due to other infections (such as bacterial infections or yeast infections)4,7,8,16,17.
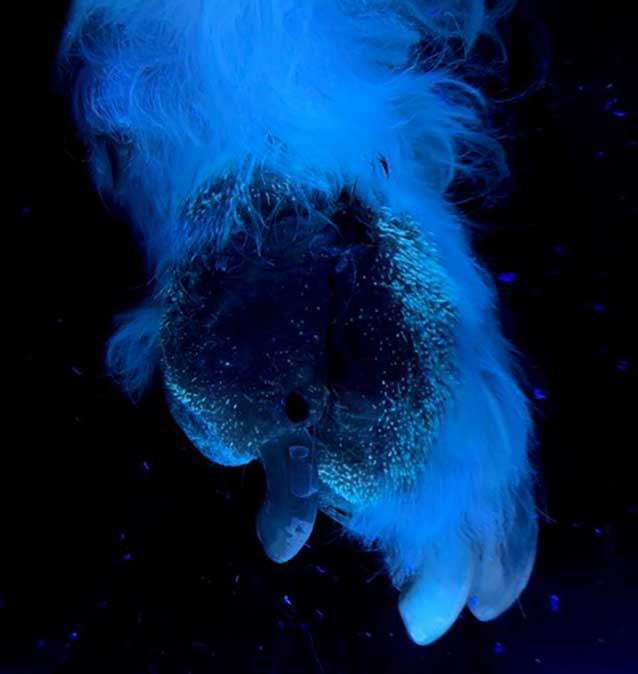
False-negative results are also possible, as not all strains of M canis fluoresce consistently, and other common dermatophytes such as Trichophyton species do not fluoresce at all, except for T schoenleinii3,7,16,17. The Wood’s lamp is considered a useful method of selecting hairs for further tests1,5; furthermore, it can be used for monitoring purposes, looking at which portion of the hair shaft the fluorescence is present.
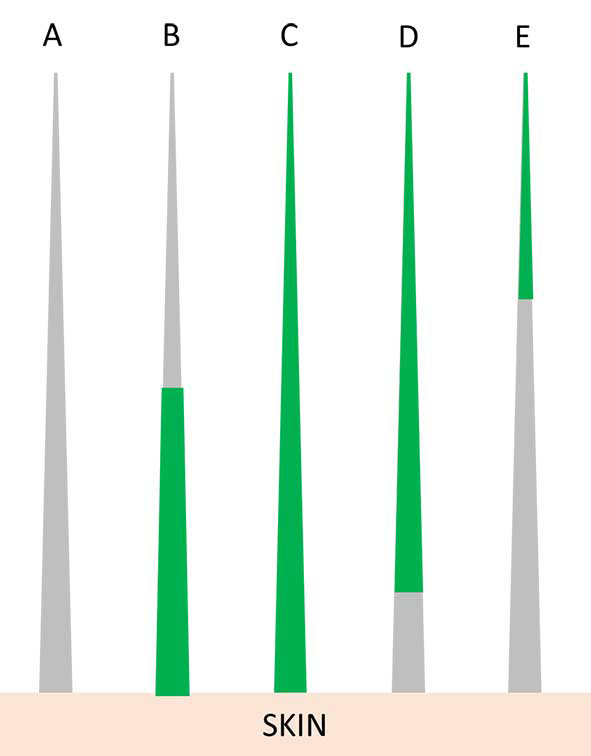
Early infected hairs show fluorescence in their base; as the infection progresses, all the hair shafts will fluoresce. When the infection has been eradicated, only the tip of the hair shaft will fluoresce. Additionally, cured animals will often have some residual glowing tip because the pigment is retained in the medulla or cortex (Figure 4)3,4,16.
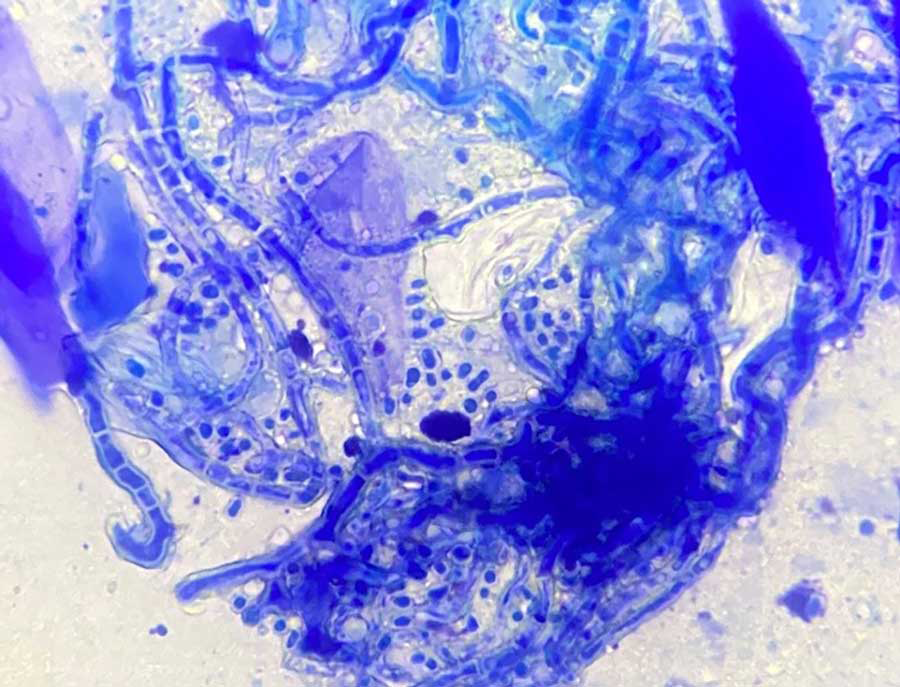
Direct microscopic examination of hair and scales is another valuable diagnostic technique, involving sample examination under a microscope for the presence of arthroconidia and fungal hyphae3-5,7,8,16,17.
Microscopy can differentiate between ectothrix (fungi that degrade the cuticle of the hair shaft) and endothrix (fungi that invade the hair shaft)3,16. The sensitivity of this method can vary depending on the experience of the examiner17,18.
The adhesive tape impression technique has also shown promise as a rapid diagnostic tool in detecting fungal elements; macroconidia are not found on cytology, but arthrospores could be observed in animals with severe infection (Figure 5)4,17.
Dermoscopy is a non-invasive technique used to examine cutaneous lesions, including those involving hair and nails3,7. It is particularly useful for examining hair follicles and skin lesions. In dermatophytosis, dermoscopy may reveal opaque, curved or broken hairs (“comma hairs”)3,4,18-20.
Fungal culture is often considered a key diagnostic tool for dermatophytosis. It involves placing hair and skin samples on a culture medium to allow for fungal growth and identification3-8,16. Several types of media are used, including dermatophyte test medium (DTM), enhanced sporulation agar (ESA) and Sabouraud’s dextrose agar (SDA)6.
DTM contains phenol red, a colour indicator, which turns red in the presence of dermatophytes, providing an early indication of potential pathogens3,4,6,7.
ESA is particularly useful for species identification based on colony colour and the morphology of macroconidia, while SDA is generally considered to provide the best overall results for fungal culture6,7. A disadvantage of fungal culture is the time required for definitive results, which can be up to 21 days, although characteristic growth often appears within the first week6.
Species identification requires expertise due to potential pleomorphism (variation in fungal morphology)3,4. It is also important to be aware that fungal cultures can yield false-positives due to environmental contamination and false-negatives due to improper sampling or the overgrowth of other, non-dermatophyte fungi3,4,6,7.
The authors’ consensus is to avoid performing dermatophyte culture in house using DTM, as fungal identification requires specific expertise in recognising the different species of dermatophytes and distinguishing between dermatophytes and non-dermatophytes moulds.
PCR testing has emerged as a valuable tool for diagnosing dermatophytosis, offering a rapid turnaround time compared to traditional culture methods3,4,6,16. Some studies have reported high sensitivity and specificity for PCR in detecting M canis and Trichophyton species6,7.
However, it is important to note that a positive PCR result does not necessarily indicate an active infection, as it can detect DNA from dead fungal organisms or from spores carried on the animal’s coat3,4,7,8. The sensitivity of PCR assays can also vary depending on the specific assay used6. A negative PCR result in a treated animal is generally considered compatible with a mycological cure4,13.
Histopathology is rarely used for the diagnosis of dermatophytosis, but can be beneficial for deep/nodular dermatophyte infections or in cases of pustular dermatosis that may resemble pemphigus foliaceus3,4,7,8,16. Stains such as periodic acid-Schiff and Gömöri’s methenamine silver can be used to visualise fungi in tissue samples4. The most common histopathologic patterns observed in dermatophytosis are:
- Perifolliculitis, folliculitis and furunculosis.
- Hyperplastic or spongiotic superficial perivascular or interstitial dermatitis with prominent parakeratotic or orthokeratotic hyperkeratosis of the epidermis and hair follicles.
- Intraepidermal pustular dermatitis (suppurative, neutrophilic epidermitis)16,21.
Given the limitations of each diagnostic approach, a strategic combination of these methods, often including Wood’s lamp examination, direct microscopy and fungal culture, with PCR serving as a useful adjunct, is typically recommended for the accurate diagnosis of dermatophytosis in dogs and cats8.
Treatment modalities
Successful management of dermatophytosis in dogs and cats typically requires a multimodal approach that includes a combination of topical and systemic antifungal therapies, along with thorough environmental decontamination8.
Topical therapy
Topical antifungal treatments (Table 3) play a crucial role in reducing the shedding of infective spores and disinfecting the hair coat3,4,5,8,16. Lime sulphur dip, applied twice weekly, is an effective topical therapy and has the advantage of residual activity on the coat2-5,16; however, this is not licensed in dogs and cats5.
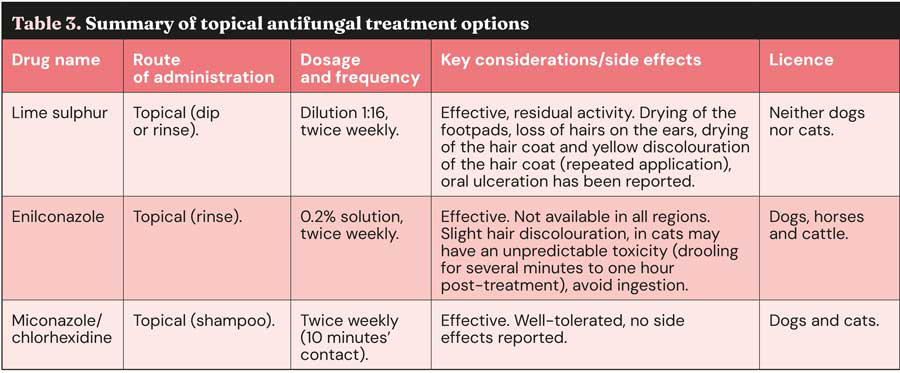
Enilconazole rinse, also applied twice weekly, is another effective option2-5,8,16. It is licensed for topical use in dogs, horses and cattle, but not in cats; anecdotal reports have stated possible toxicity, but limited experimental work has failed to reproduce these effects5. Shampoo containing a combination of miconazole and chlorhexidine used twice weekly is also commonly recommended2-5,8,16. This is the only topical product licensed for dermatophytosis treatment in cats5.
Other topical agents, such as ketoconazole, miconazole, climbazole, terbinafine and accelerated hydrogen peroxide have shown promise in in vitro studies, but require further in vivo research to definitively establish their efficacy3. Chlorhexidine used as a monotherapy is generally considered poorly effective against dermatophytes2,3.
While clipping the hair around lesions was once a routine recommendation, whole-body clipping is now being reconsidered due to the stress it can cause and the potential for microtrauma that may worsen the infection3,4,8. Furthermore, clipping can spread infection to unaffected areas3,4. If a patient needs to be clipped, this should ideally be performed using scissors, and removing the matted hairs is recommended only to facilitate topical product penetration3,4.
Systemic therapy
Systemic antifungal therapies are often necessary to target the fungus within the hair follicles.
Itraconazole, administered orally at a dose of 5mg/kg daily or using a pulse therapy regimen (one week on, one week off), is highly effective and generally well tolerated in both dogs and cats2-5,8,16. It is crucial to use commercially available, non-compounded formulations of itraconazole due to the poor bioavailability of compounded products8. Itraconazole is a systemic antifungal licensed in cats5; however, human itraconazole can be used in dogs, following the cascade.
Terbinafine, given orally at a dose of 20mg/kg to 40mg/kg daily or using pulse therapy, is another very effective systemic antifungal agent2-5,8,16. Orally, this antifungal is available only as a human formulation and is not licensed for use in dogs and cats.
Ketoconazole is a systemic azole licensed only in dogs. It is administered orally at 5mg/kg to 10mg/kg once or twice daily. It is effective, but carries a higher risk of potential side effects, and is generally considered less safe than itraconazole or terbinafine3-5,8,16.
Griseofulvin, while effective, has a greater potential for adverse effects compared to itraconazole and terbinafine. Griseofulvin is no longer available as a veterinary formulation for small animal use, as safer alternatives are now available2,3,5,8,16. It is also contraindicated in young kittens (less than six weeks) and pregnant animals5.
Fluconazole has poor activity against dermatophytes and is, therefore, not recommended for treating this infection3,5,8,16; furthermore, this azole agent is not licensed for use in small animals.
Contrary to what was previously hypothesised22, lufenuron has been shown to be ineffective against dermatophytes and is not recommended3,8,16.
Because of the azole effect on the cytochrome P450, it is important to consider drug interactions and decrease doses of other medications if their metabolism is affected by this interaction (such as ciclosporin)8,16.
Detailed information on systemic antifungal treatments, including dosage, mechanisms of action and side effects, are reported in Table 4.
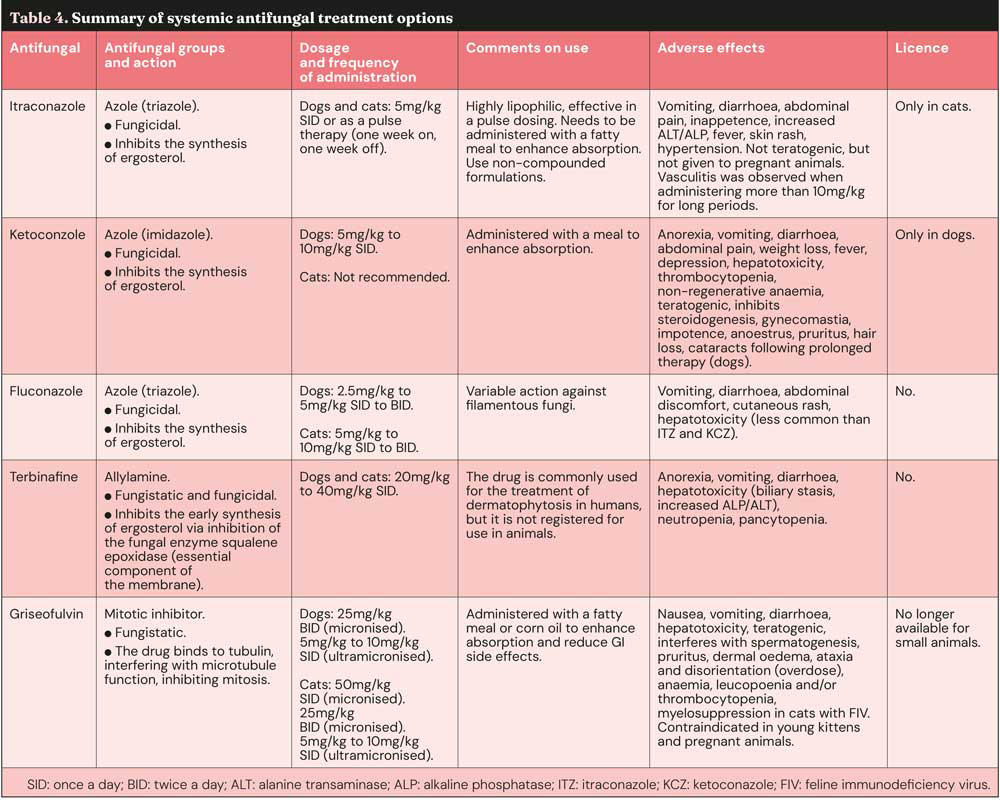
It is crucial to continue antifungal treatment until a mycological cure is achieved, which is typically defined as having at least one, and preferably two, negative fungal cultures or PCR tests collected after the resolution of clinical signs8,16. It is important to remember that clinical resolution of the lesions does not always equate to the eradication of the fungus, and premature cessation of treatment can lead to recurrence of infection or resistance4,13.
Environmental management and prevention
Environmental decontamination is a critical component of managing and preventing the spread of dermatophytosis in both household and multi-animal settings3-5,8.
Dermatophyte spores can survive in the environment for extended periods, making thorough disinfection essential to prevent reinfection and transmission3,5,7,8.
Recommended strategies for environmental disinfection include frequent and thorough mechanical removal of debris and hair through vacuuming and cleaning of all surfaces3,4,8. Surfaces should be washed with detergent and then rinsed. The use of antifungal disinfectants is also highly recommended. Effective options include from 1:10 to 1:100 diluted sodium hypochlorite (bleach), enilconazole spray or fogger at 20µL/L (where available), accelerated hydrogen peroxide, potassium peroxymonosulphate, quaternary ammonium compounds, and lactic acid3,4,8. Contaminated laundry, such as bedding and towels, should be washed thoroughly in a washing machine; studies have found no difference between washing at 30°C or 60°C; however, two washes on the longest wash cycle were effective to eliminate fungal spores3,8. Carpets and upholstery can be cleaned with carpet shampooers or hot water extraction, potentially after pre-treatment with an antifungal disinfectant1,3.
During the treatment period, it is advisable to isolate infected animals in areas that can be easily cleaned and disinfected2,3,8. Confinement should be considered with care and for a short period, given the possible behavioural issues that may arise from a prolonged period of isolation and no socialisation in kittens or puppies3,4.
When handling infected animals, it is important to take appropriate hygiene measures, such as wearing gloves and washing hands thoroughly afterwards, to prevent the spread of the fungus to humans23.
Zoonotic aspects of dermatophytosis
Dermatophytosis is a significant zoonotic disease, meaning it can be readily transmitted between animals and humans1,3,4,5,7,8,24.
Humans can contract dermatophyte infections through direct physical contact with infected animals or indirectly through contact with environments or objects (fomites) contaminated with fungal spores1,25.
M canis, the most common cause of dermatophytosis in cats and frequently in dogs, is also a major zoonotic dermatophyte, readily transmissible to humans1,25. Other dermatophyte species, such as T mentagrophytes and M gypseum, are also zoonotic and can be transmitted from animals to humans1,25. Certain populations, including young children, the elderly and individuals with compromised immune systems, are considered to be at a higher risk of contracting zoonotic dermatophytosis1.
Given the potential for human infection, veterinary involvement is crucial in cases where zoonotic transmission of dermatophytosis is suspected3,4. It is important to recognise that even animals that do not show any clinical signs of dermatophytosis can still carry the fungus and contaminate the environment, thereby posing a risk to humans and other animals in the household3-5,8,16.
The zoonotic nature of dermatophytosis emphasises the importance of prompt and accurate diagnosis, effective treatment and diligent environmental management in affected dogs and cats to safeguard public health1,3,5,7. Veterinarians play a vital role in educating pet owners about the zoonotic risks associated with dermatophytosis and in guiding preventive measures to protect themselves and their families3.
Antifungal resistance
While fungal infections were once easily treated, the incidence of human severe systemic fungal infections has increased – particularly in patients with compromised immune systems15. This has led to an increase in drug resistance in pathogenic fungi, including dermatophytes15.
Historically, the clinical resistance of microbes has been defined as the persistence or progression of an infection despite appropriate antimicrobial therapy15. In vivo, resistance is also correlated with antifungal misuse because patients often fail to finish the full course of treatment. Therefore, the inadequate use or dosage of drugs contributes to the failure in eliminating the disease agent completely, encouraging growth of the most resistant strains, which may lead to hard to treat fungal infections15.
The in vitro resistance of an isolate can be classified as either intrinsic or acquired. Intrinsic resistance allows all normal members of a species to tolerate a particular drug; in this case, a specific characteristic responsible for resistance is inherent to the species and has arisen through the process of evolution. Acquired resistance is a term used when a resistant strain emerges from a population that was previously drug sensitive15. Various biochemical mechanisms contribute to the phenotype of drug resistance in fungi. The most frequent ones involve a decrease in drug uptake, structural alterations in the target site, and an increase in drug efflux or intracellular target levels15. Only a few reports have addressed the drug resistance mechanism in dermatophytes, and most of them have been described in Trichophyton rubrum. It is noteworthy that resistance to a particular drug can be achieved by more than one mechanism, and probably, under certain circumstances, they are activated simultaneously15.
Conclusion and future directions
Dermatophytosis remains a prevalent superficial fungal infection in dogs and cats, with significant implications for both animal and human health due to its zoonotic potential. This review has highlighted the key aspects of this disease, including its aetiology, epidemiology, clinical presentations that can mimic other dermatological conditions, the complex interplay of pathogenesis and host immunity, the array of diagnostic tools available and the current treatment strategies involving topical and systemic antifungals, along with environmental management. The lack of a single definitive diagnostic test underscores the importance of a comprehensive approach, integrating clinical findings with appropriate diagnostic testing to confirm active infection.
Treatment success relies on a combination of topical and systemic therapies administered until mycological cure is achieved, coupled with diligent environmental decontamination to minimise the risk of reinfection and transmission.
The zoonotic nature of dermatophytosis necessitates that veterinary professionals educate pet owners about the potential risks.
The potential for antifungal resistance in dermatophytes also warrants continued monitoring and research.
- This article appeared in Vet Times (2025), Volume 55, Issue 32, Pages 5-12
Authors
Davide Immediato graduated from the University of Bari Aldo Maro, Italy, in 2013. In 2014, he started his PhD in “animal health and zoonosis” at the same university, where he worked in medical mycology and parasitology. In 2017, he moved to the UK where he worked for approximately three years in first opinion practices. In 2019, Davide joined Southfields Veterinary Specialists to complete a one-year rotating internship and in 2020, he was awarded the title of GPCert in dermatology. He joined Davies Veterinary Specialists in 2021 as a clinician and from October 2023, he became a resident of the European College of Dermatology.
Filippo De Bellis qualified from the University of Bari Aldo Maro in 2001. In 2006, he joined the RVC as a resident in veterinary dermatology, gaining the RCVS certificate in veterinary dermatology in 2009 and becoming a diplomate of the European College of Veterinary Dermatology in 2010. Filippo is head of dermatology services at Davies Veterinary Specialists and London Vet Specialists.
References
- 1. Paryuni AD, Indarjulianto S and Widyarini S (2020). Dermatophytosis in companion animals: A review, Vet World 13(6): 1,174-1,181.
- 2. Moriello KA (2004). Treatment of dermatophytosis in dogs and cats: review of published studies, Vet Dermatol 15(2): 99-107.
- 3. Moriello KA, Coyner K, Paterson S and Mignon B (2017). Diagnosis and treatment of dermatophytosis in dogs and cats. Clinical Consensus Guidelines of the World Association for Veterinary Dermatology, Vet Dermatol 28(2): 266-e68.
- 4. Moriello KA (2019). Dermatophytosis in cats and dogs: a practical guide to diagnosis and treatment, In Pract 41(4): 138-147.
- 5. De Bellis F (2011). Dermatophytosis in cats and dogs: signs and management, Vet Times, tinyurl.com/bdcp9b7h
- 6. Cheung W, Ellis A, Idle A et al (2024). Retrospective comparison of polymerase chain reaction and culture-based identification for diagnosis of Microsporum canis and Trichophyton Spp. in shelter cats and dogs, J Shelter Med Community Anim Health 3(1): 1-7.
- 7. Moskaluk AE and VandeWoude S (2022). Current topics in dermatophyte classification and clinical diagnosis, Pathogens 11(9): 957
- 8. Marsella R (2021). Dermatophytoses in dogs and cats, TVP, tinyurl.com/4vbztftp
- 9. Bajwa J (2020). Feline dermatophytosis: Clinical features and diagnostic testing, Can Vet J 61(11): 1,217-1,220.
- 10. Baert F, Stubbe D, D’hooge E et al (2020). Updating the taxonomy of dermatophytes of the BCCM/IHEM collection according to the new standard: A phylogenetic approach, Mycopathologia 185(1): 161-168.
- 11. Gräser Y, Monod M, Bouchara J-P et al (2018). New insights in dermatophyte research, Med Mycology 56(Suppl 1): 2-9.
- 12. Lopes R, Garcês A, Silva A et al (2024). Dermatophytosis in companion animals in Portugal: a comprehensive epidemiological retrospective study of 12 years (2012–2023), Microorganisms 12(8): 1,727
- 13. Tainwala R and Sharma Y (2011). Pathogenesis of dermatophytoses, Indian J Dermatol 56(3): 259-261.
- 14. Martinez-Rossi NM, Peres NTA and Rossi A (2008). Antifungal resistance mechanisms in dermatophytes, Mycopathologia 166(5-6): 369-383.
- 15. Moriello KA (2021). Dermatophytosis. In Jackson HA and Marsella R (eds), BSAVA Manual of Canine and Feline Dermatology (4th edn), BSAVA, Gloucester.
- 16. Miller WH, Griffin CE and Campbell KL (2013). Muller and Kirk’s Small Animal Dermatology (7th edn), Saunders, St Louis.
- 17. Silveira Mendes AL, de Azevedo MI and Pimenta da Costa-Val Bicalho A (2024). Dermatophytosis in cats: A comprehensive study on diagnostic methods and their accuracy, Open Vet J 14(4): 1,072-1,075.
- 18. Ludwig BC, Tyler SA, Lima T and Vogelnest LJ (2024). A prospective study evaluating the adhesive tape impression for the diagnosis of dermatophytosis in dogs and cats, Vet Dermatol 35(6): 694-703.
- 19. Scarampella F, Zanna G, Peano A et al (2015). Dermoscopic features in 12 cats with dermatophytosis and in 12 cats with self-induced alopecia due to other causes: an observational descriptive study, Vet Dermatol 26(4): 282-e63.
- 20. Scarampella F, Zanna G and Peano A (2017). Dermoscopic features in canine dermatophytosis: some preliminary observations, Vet Dermatol 28(2): 255-257.
- 21. Gross TL, Ihrke PJ, Walder EJ and Affolter VK (2005). Skin Diseases of the Dog and Cat: Clinical and Histopathologic Diagnosis (2nd edn), Blackwell, Oxford.
- 22. Ben-Ziony Y and Arzi B (2000). Use of lufenuron for treating fungal infections of dogs and cats: 297 cases (1997–1999), J Am Vet Med Assoc 217(10): 1,510-1,513.
- 23. Bagra JK, Nair SS, Athira VS et al (2022). Dermatophytosis in canines and felines, Sci World 2(6): 661-669.
- 24. Gupta AK, Wang T, Susmita et al (2025). Global dermatophyte infections linked to human and animal health: A scoping review, Microorganisms 13(3): 575.
