16 Sept 2025
Feline atopic skin syndrome
Victoria Robinson BVM&S, Sc, CertAVP(VD), DipECVD, MRCVS and Matt McHale BVetMed, MRCVS discuss signs, diagnosis and management of this allergic disease in cats

A Devon rex. Image: Ermolaev Alexandr / Adobe Stock
Feline atopic skin syndrome (FASS) is the new name given to allergic skin disease in cats (Santoro et al, 2021). Previously, many different names were given to this disease, including the unwieldy non-flea, non-food hypersensitivity dermatitis, as well as the misnomer of feline atopic dermatitis (Hobi et al, 2011).
It is a lifelong disease that requires regular management to prevent flares – in this sense, it is similar to eczema in humans and atopic dermatitis in dogs, with some distinct differences; for example, skin barrier dysfunction has so far not been demonstrated in FASS (Marsella and De Benedetto, 2017).
FASS is a complex condition that is not fully understood.
Factors include:
- Abnormal skin inflammation.
- Allergies to environmental allergens (for example, house dust mites and pollens), although some cats do not have any allergic factors.
- Secondary skin and ear infections, although these are much less common than in dogs with atopic dermatitis and humans with atopic eczema.
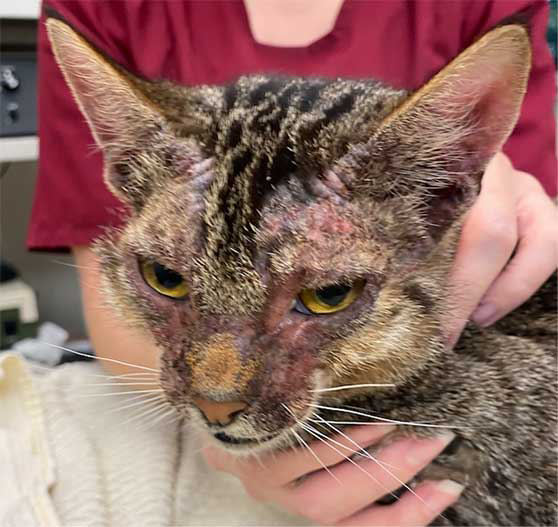
FASS can present in a clinically indistinguishable way to feline food allergy (FFA), flea-allergic dermatitis (FAD) and dermatitis associated with other skin parasites and behavioural problems.
It is vital to rule out FAD and FFA as part of a thorough work-up of feline pruritus (Favrot et al, 2014). It may also be necessary to rule out other diseases in some cats. Stress, anxiety and other behaviour problems can exacerbate itch and inflammation in some cats (especially in multi-cat households); however, they are not the sole component for disease development.
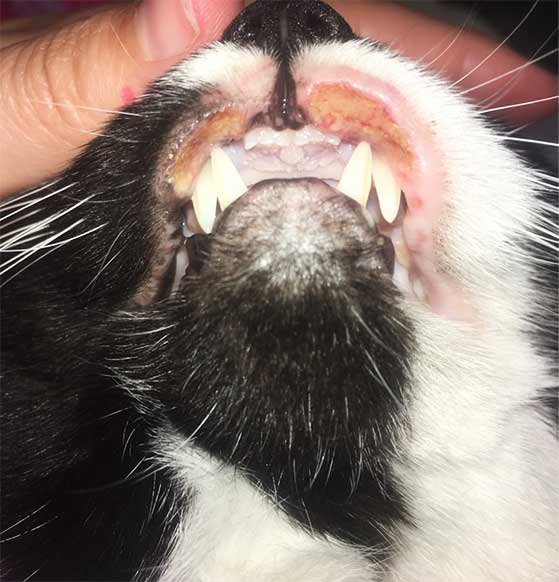
FASS fits under the umbrella term of “feline atopic syndrome” (FAS). This encompasses the three currently recognised atopic manifestations: cutaneous, gastrointestinal and respiratory.
FFA also fits under this umbrella term, with FAD outside of the umbrella, but clinically indistinguishable from FASS and FFA.
A cat presenting with cutaneous, gastrointestinal or respiratory signs should have FAS among the differential diagnosis list where clinically appropriate.
Which cats are affected by FASS?
- Prevalence. FASS is estimated to affect up to 10% to 20% of cats (Marsella and De Benedetto, 2017).
- Age. The age of onset varies widely, and FASS is seen in cats as young as six months old up to 15 years old. However, most cats start between six months and five years of age (Santoro et al, 2021).
- Breed predisposition. Less is known about the breed predilection in cats compared to dogs. However, evidence exists that the Abyssinian, Maine coon, Devon rex, Persian, Himalayan, Somali and ocicat breeds are predisposed to FASS (Ravens et al, 2014; Scott and Miller, 2013; Hobi et al, 2011). Despite this, FASS can affect any breed, including domestic breeds.
- Sex predilection. Female cats are slightly more likely to develop FASS than male cats (Hobi et al, 2011; Ravens et al, 2014).
What are the signs of FASS?
Cats generally present with one or more of the following characteristic skin lesions (Santoro et al, 2021):
- Face/head/neck pruritus – this involves excessive rubbing or scratching, which can cause erythema, lichenification (thickening) of the skin, alopecia and crusting. This may also cause ocular issues if the cat traumatises the eyes or eyelids themselves.
- Eosinophilic granuloma complex is a group of clinical manifestations:
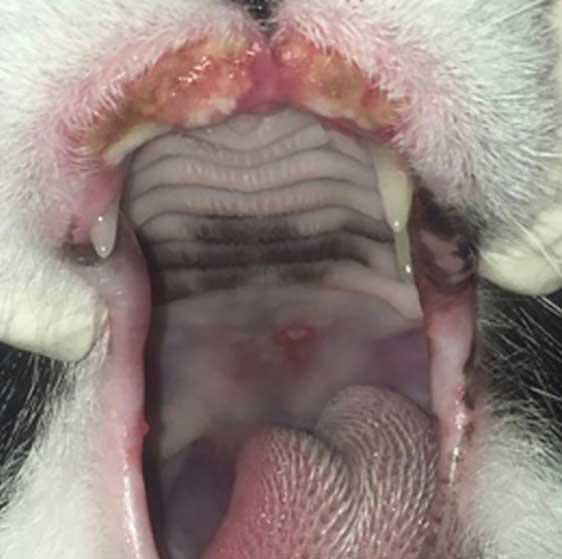
- Indolent ulcers on the lips.
- Eosinophilic plaques (mostly on the abdomen or medial limbs), which are generally intensely pruritic.
- Eosinophilic granulomas, which can be on the skin and/or in the mouth and are often non-pruritic.
- Self-induced alopecia, which is most commonly seen on the ventral abdomen, but can be on any part of the body the cat can reach. This is caused by excessive licking, biting or scratching. The owner may not witness this behaviour, as some cats can be very secretive.
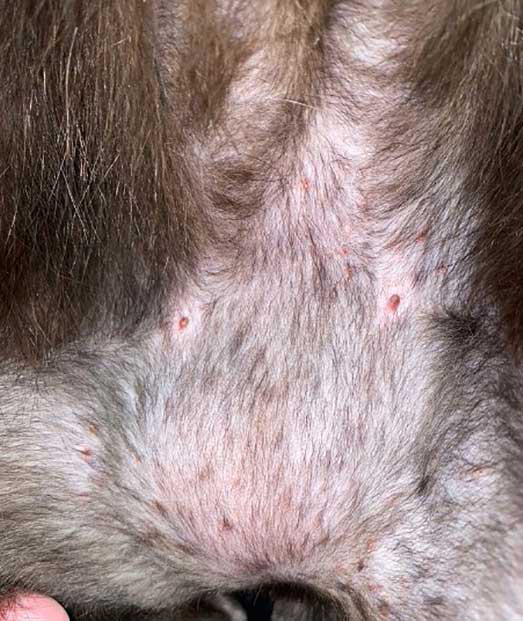
- Miliary dermatitis presents as numerous small papules with associated crusting, mainly on the body.
The severity of clinical signs may vary from very mild to intense, depending on the cat and how long signs have been evident. FASS usually presents with itching (scratching, licking, biting, rubbing, rolling and/or headshaking) that can be generalised or more localised to the ears, face, eyes, axillae, inner thighs, paws and/or abdomen. It is important to recognise these reactions patterns and appreciate that if patients present with other lesions, then FASS is less likely and other differentials and investigations should be considered.
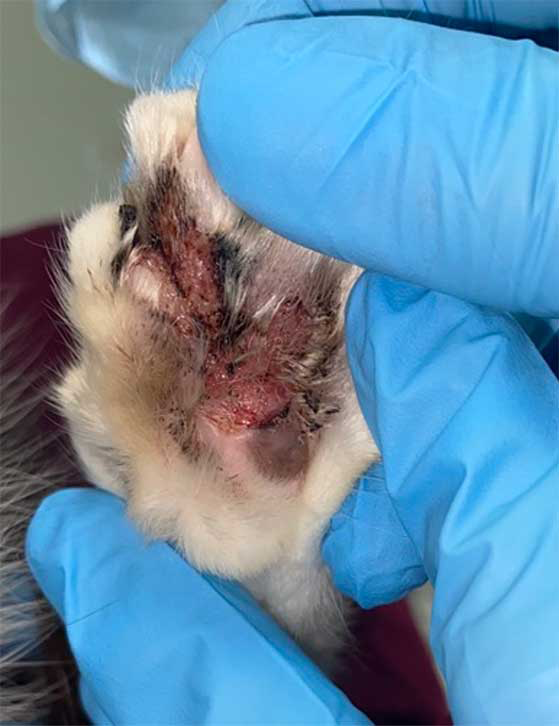
How do we diagnose cats with FASS?
The clinical signs of FASS can look similar to FAD, FFA and other conditions.
In addition, no specific diagnostic test exists for FASS; therefore, similarly to canine atopic dermatitis, the diagnosis is based on the history, clinical signs and ruling out other possible skin diseases for each cat (especially FAD and FFA).
We first assess for evidence of secondary infections and also rule out conditions that may present in a similar manner to FASS, FFA and FAD, such as other ectoparasite infestations.
Assessing for secondary infection and inflammatory cell population
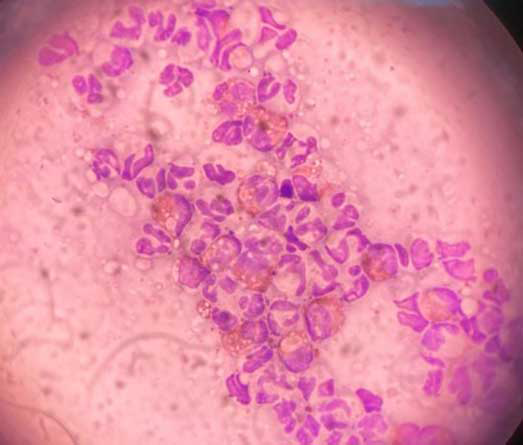
Performing cytology on lesions is vital to characterise the microbial populations present and their relative abundance.
Tape strips and direct impression smears are mainstays of cytology. Tape strips can be utilised on drier lesions after the removal of scale or dried crust, if present.
Direct impression smears, or collecting a sample using a cotton bud then rolled on to a microscope slide, should be used on more exudative lesions.
Cytology will also allow for analysis of the inflammatory cells present. In cats with FASS, we would anticipate eosinophils and neutrophils when sampling the classic reaction patterns. If other cells are present, this may suggest less common differentials; for example, an exudative cutaneous lymphocytosis may show predominantly lymphocytic inflammation, with fewer neutrophils and eosinophils.
Secondary infection or microbial overgrowth is not specific to FASS, and is also less common with FASS than it is in canine atopic dermatitis. The feline microbiome is not as well researched as the human or canine microbiome. However, evidence suggests that cats with FASS have higher relative abundances of Staphylococcus species on their skin (Older et al, 2017). Bacterial folliculitis, as seen in dogs, is not recognised in cats. In the majority of cats, secondary infection is associated with self trauma and translocation of microbes to an eroded or ulcerated area.
Malassezia dermatitis is an uncommon finding in cats, and may be suggestive of significant immunocompromise. It may be seen with thymoma-associated exfoliative dermatitis and paraneoplastic syndromes (Godfrey, 1998; Forster-Van Hijfte et al, 1997). Malassezia species is also more abundant on the skin of cats infected with FIV or FeLV (Sierra et al, 2000). Sphynx and Devon rex cats are known to have higher relative abundances of Malassezia species, and so Malassezia dermatitis is more common and less concerning in these breeds (Volk et al, 2010; Ahman and Bergström, 2009).
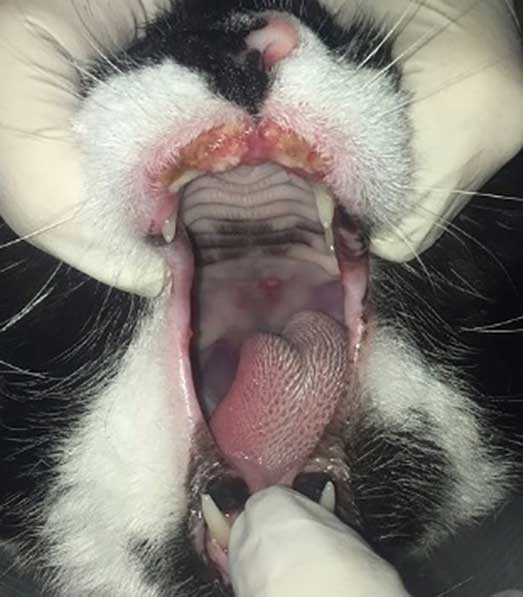
Investigating for ectoparasite infestation
Assessing for non-flea ectoparasite infestation is often required in the case of pruritic cats.
Ectoparasites of cats include:
- Cheyletiella blakei.
- Demodex gatoi (transmissible) or Demodex cati (non-transmissible).
- Lice.
- Neotrombicula autumnalis (harvest mites).
- Notoedres cati (feline scabies) – very rare.
- Otodectes cynotis (ear mites).
Classic presentations of these infestations differ in terms of predilection sites and clinical signs. Each can be ruled out quickly with a number of in-house tests. Combing the coat, taking ear swabs, hair plucks or performing skin scrapes may be carried out quickly and cheaply to assess for the presence of these ectoparasites.
Seasonal patterns can also be used to narrow down the possible differential diagnoses; for example, Neotrombicula autumnalis is uncommon outside of the months of August, September or October in the UK. The same may not be assumed for other areas of the world, however, with perennial activity in tropical and subtropical climates.
Any identified ectoparasite infestation should be managed accordingly. International guidelines for their management may be found through the European Scientific Counsel Companion Animal Parasites (www.esccap.org).
Investigating for FAD
To rule out FAD, we use isoxazolines, as these kill fleas and mites quickly and effectively. Fluralaner has been shown to reduce flea levels on cats and dogs by more than 99% in seven days and 100% at week 12, without adjuvant therapies and environmental controls (Dryden et al, 2018).
Environmental controls are often not needed, as isoxazolines affect almost all life stages of the flea cycle. Williams et al (2014) demonstrated that surviving fleas did not produce eggs by day four to five, with fast kill of adults and significant reductions in pupal development. All animals in the household must be treated to ensure that the flea burden is as low as possible.
Long-acting flea preventive medication such as tigolaner (13 weekly) spot-on or fluralaner (12 weekly) spot-on are useful to rule out FAD, as reapplication should not be necessary in the initial diagnostic phase. As environmental controls are rarely, if ever, necessary, when using isoxazolines, as a team we are now able to forego the use of household sprays containing permethrin and other ecotoxic ingredients.
No “gold standard” test for FAD exists, apart from flea chamber testing, which is only possible in research settings. Confirming or ruling out FAD is most easily done by using long-acting isoxazolines, as described previously.
Investigating for FFA
Investigating FFA requires an elimination diet trial for 8 to 12 weeks.
Two diets have been proven to be effective for food trials: Purina HA (Jackson et al, 2003) and Royal Canin Anallergenic (Olivry et al, 2017). Diets containing chicken hydrolysate may not be suitable for diet trial based on previous studies in dogs, in which 40% of chicken-allergic dogs flared when fed a diet consisting of moderately hydrolysed chicken liver (Bizikova and Olivry, 2016).
However, other diets (including home-cooked novel proteins), may be suitable for some cats, alongside advice from a veterinary nutritionist, to ensure the diet is balanced. It is vital to ascertain which proteins a cat has eaten or had access to; for example, rabbit, kangaroo and ostrich-based treats are becoming more common – if a cat has eaten these treats, these proteins would no longer be novel.
Cross-reactions and co-sensitisation between ingredients may also be a significant consideration (Bexley et al, 2017). Chicken is likely to cross-react with turkey and duck, as well as other waterfowl and likely gamebirds. Chicken will also cross-react with raw egg, and may cross-react with cooked egg in a number of cases. Beef is likely to cross-react with lamb, venison and other ruminants. Ruminants are descended from a closer common ancestor to whales than even pigs, and so cross-reactions between pork and beef are less likely.
Novel protein trials may be a more attainable method of testing cats for food allergy due to increased palatability. The authors warn to monitor any feline patient closely and regularly during an elimination diet.
Regular weight checks are vital – particularly in multi-cat households, where starvation may not be noted as readily.
No reliable blood, saliva or hair test is available to identify food allergy, and no evidence base exists to support the use of serology in the diagnosis of food allergy (Olivry et al, 2015).
How do we manage cats with FASS?
FASS is a lifelong disease. It requires ongoing proactive management to maintain remission and prevent flares. It is a manageable disease, but it is very rarely curable.
Regular flea control is normally advised, as any fleas can make the skin problem much worse. However, if FAD is ruled out and risk of exposure to ectoparasites is minimal, then flea control should not be necessary – especially when, as a profession, we should be more cognisant of the impact of endectocides on insects other than the targeted population.
Most cats need a multimodal approach with more than one type of treatment. This is usually more effective and safer than relying on a single treatment.
In broad terms, we use a two-phase approach:
- Induction therapy to initially bring the itch and inflammation under control.
- Systemic or topical glucocorticoids.
- Ongoing proactive maintenance therapy to prevent relapses.
- Ciclosporin.
- Topical glucocorticoids.
- Allergen-specific immunotherapy (ASIT).
- Gabapentin.
- Maropitant.
- PEAum.
- Skin barrier care.
- Pheromones/mood modifying medications.
- Systemic glucocorticoids (last resort maintenance therapy, used at lowest effective dose and frequency).
ASIT involves giving small volumes of allergens regularly to induce tolerance so that, with time, the cat does not react to them in the same way. This is the only therapy available that modifies the response to environmental allergens; all other available treatments deal with the consequences of the response to allergens.
Long term, it may be the most economical option for the management of FASS.
Intradermal allergen testing and/or allergen immunoglobulin E serology should not be used to diagnose FASS. Prior to performing allergen testing, it is vital to assess patient temperament and suitability to the preferred route of administration (most commonly subcutaneous). It is also vital to have the owner understand the commitment and costs prior to testing, as some owners may not be able to schedule such regular revisit appointments – especially with outdoor cats.
Allergen testing should be used to identify positive allergens that may be used to formulate ASIT. The results of allergen testing should be interpreted along with clinical history. If positive results do not match the seasonality or clinical picture, those allergens should not be included in immunotherapy.
Seasonal allergens can be checked using pollen calendars due to changes due to climate and regional differences across the UK. Example pollen calendars and forecasts can be found through the University of Worcester and Kleenex websites.
Perennial allergens such as house dust mites may still present with a seasonal flare; for example, when the windows are closed and heating is turned on. House dust mites may also be present in higher numbers based upon specific household activities, such as DIY and Christmas decorating. Moulds may be present year round, with a seasonal flare due to increased levels in the environment in early autumn as trees drop their leaves.
Allergen testing is only useful if you plan on pursuing long-term ASIT – it is not diagnostic for FASS, as we see some false-positive tests in cats with other skin diseases, and some cats have a non-allergic form of FASS. In addition, practical allergen avoidance is very difficult.
When formulating ASIT, likely false positives should not be included; for example, a cat only flaring in winter should not have grass pollens included.
Cross-reactive allergens should also be assessed and the strongest positive should be included; for example, a cat with positive results for meadow fescue, orchard and perennial rye grass is reacting only to the Festucae tribe of grasses (Jones et al, 1995), and only requires one of these three allergens to be included in its ASIT.
Anti-inflammatory treatments such as systemic or topical glucocorticoids (steroids) and ciclosporin are a mainstay in the management of FASS.
Systemic glucocorticoids are most commonly used in the induction phase of treatment. Prednisolone at 2mg/kg once daily is often necessary to control itch and reverse chronic inflammation in the skin – particularly with eosinophilic granuloma complex. This should be weaned to assess for rebound itch prior to full cessation.
Dexamethasone injectable solution may be used by mouth at 0.2mg/kg once daily. Dexamethasone should not be used as a first-line treatment, as it is not licensed and due to the increased possibility of diabetogenesis when compared to prednisolone. The likelihood of significant adverse effects in the first month of treatment is low, however (McClintock et al, 2021).
Topical glucocorticoids including hydrocortisone aceponate may be used with localised disease as an induction agent or maintenance therapy.
Use of each product should be tapered to the lowest effective application frequency, to limit side effects associated with topical steroid application, such as cutaneous atrophy, ulceration, telangiectasia, alopecia, comedones, calcinosis cutis and altered hypothalamic-pituitary-adrenal axis function (Nuttall et al, 2009; Thomas et al, 1999).
Ciclosporin is an excellent therapy in the maintenance phase of FASS management. Due to a delayed onset of three to four weeks, it is not recommended as a sole therapy in the induction phase of treatment, and should be used alongside rapidly acting therapies such as concurrent glucocorticoids in the lag phase of treatment.
A dose of 7mg/kg once daily is recommended (Roberts et al, 2016a). Ciclosporin may be tapered once clinical remission is achieved, with twice-weekly dosing being sufficient in many cases (Roberts et al, 2016b).
In more severe cases, tapering by one day per week (reducing from seven to six days per week) every six weeks may be necessary. We will often start patients on ciclosporin with concurrent prednisolone at 1mg/kg to 2mg/kg once daily for one to three weeks before reducing to every other day for one to two weeks and stopping prednisolone, allowing ciclosporin to continue.
Testing for FIV/FeLV with or without Toxoplasma species is recommended prior to initiation of ciclosporin treatment alongside biochemistry and haematology (Colombo and Sartori, 2018). Early evidence suggests the use of maropitant as an antipruritic medication in cats. This medication is thought to work by blocking NK-1 receptors, which are involved in the transmission of substance P, a neuropeptide associated with inflammation and itch (Maina and Fontaine, 2019).
This study assessed response to maropitant over one month, with a dose range of 1.9mg/kg to 2.4mg/kg once daily. Efficacy and tolerability were judged as good to excellent by 83% of clients (Maina and Fontaine, 2019).
PEAum has been shown to reduce pruritus scores when compared to a placebo control, for maintenance of FASS in cats achieving remission with methylprednisolone (Noli et al, 2019). In an uncontrolled study, pruritus, erythema and alopecia improved in 64% of cats, with two-thirds showing improvement of eosinophilic dermatitis lesions (Scarampella et al, 2001). Both show PEAum to be safe, with gastrointestinal side effects occurring in placebo-treated cats at similar rates. Zoetis has recently recommended not to use Apoquel (oclacitinib) in cats due to safety concerns. If considering use of a JAK inhibitor in a feline patient, the authors recommend contacting a dermatology specialist to discuss this.
What other therapies are used to manage FASS?
Skin barrier care with emollient or antimicrobial shampoos, foams, pads and rinses (if tolerated), emollient spot-on preparations, and essential fatty acid supplements can be used as adjuvant therapies (Dropsy et al, 2024).
Behaviour modification may be necessary in cats where stress or anxiety exacerbates their itch or inflammation (as in human eczema). This can include adjusting their lifestyle, using feline pheromones and/or mood modifying drugs.
Commonly, initial therapy consists of gabapentin at approximately 10mg/kg twice daily, with scope to increase if necessary. Assessing the home dynamic in multi-pet households can be vital; for example, ensuring water and food stations are separate to reduce stress.
Water and food stations should outnumber the cat population by one or more, as should the number of litter trays. Allowing cats to get up high and to hide is important to avoid fear, anxiety and stress. Adapting the home to better cater for anxious cats can be vital in the management of cats with FASS.
Summary
- FASS is a complex condition that is clinically indistinguishable from FFA and FAD.
- Thorough history taking and examination is vital in every case of feline pruritus.
- Cytology is helpful in narrowing differentials, assessing for microbial overgrowth and streamlining clinical planning.
- Proactive management of inflammation can reduce costs, improve patient outcomes and reduce the need for glucocorticoids and antimicrobials long term.
- For complex cases, referral should be sought promptly, as this is likely to lead to increased patient welfare, increased owner satisfaction and often reduce financial outlay long term.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
- This article appeared in Vet Times (2025), Volume 55, Issue 37, Pages 6-13
- Dermatophytosis in dogs and cats – a comprehensive review – an article from August 2025
Authors
Victoria Robinson graduated from The Royal (Dick) School of Veterinary Studies (R[D]SVS)in 2008. After several years in general practice, she completed a rotating internship at the University of Liverpool before completing residency training with Hilary Jackson and Peter Forsythe at the Dermatology Referral Service in Glasgow. She re-joined The R(D)SVS as senior lecturer in veterinary dermatology in 2022.
Matt McHale graduated from the RVC in 2019 and worked in busy small animal practice for three years, prior to joining The Royal (Dick) School of Veterinary Studies in Edinburgh. He started as an intern in veterinary dermatology before starting his residency in March 2023. Matt has a keen interest in all areas of veterinary dermatology – especially client communication and compliance in atopic disease and student teaching.
References
- Ahman SE and Bergström KE (2009). Cutaneous carriage of Malassezia species in healthy and seborrhoeic Sphynx cats and a comparison to carriage in Devon rex cats, Journal of Feline Medicine and Surgery 11(12): 970-976.
- Bexley J, Nuttall TJ, Hammerberg B and Halliwell RE (2017). Co-sensitization and cross-reactivity between related and unrelated food allergens in dogs – a serological study, Veterinary Dermatology 28(1): 31-e7.
- Bizikova P and Olivry T (2016). A randomized, double-blinded crossover trial testing the benefit of two hydrolysed poultry-based commercial diets for dogs with spontaneous pruritic chicken allergy, Veterinary Dermatology 27(4): 289-e70.
- Colombo S and Sartori R (2018). Ciclosporin and the cat: Current understanding and review of clinical use, Journal of Feline Medicine and Surgery 20(3): 244-255.
- Dropsy H, De Jaeger X, Cozar A, Billy C, Bressolin A, Briand A, Debraine M, Deschamps V, Noli C, Puozzo-Barichard A and Gatellet M (2024). Performance of applications of Ophytrium-containing mousse with or without shampoo in cats with pruritic and irritated skin: a multicentre prospective field trial, Journal of Feline Medicine and Surgery 26(9): 1098612X241264718.
- Dryden MW, Canfield MS, Bocon C, Phan L, Niedfeldt E, Kinnon A, Warcholek SA, Smith V, Bress TS, Smith N, Heaney K, Royal C, Normile D, Armstrong R and Sun F (2018). In-home assessment of either topical fluralaner or topical selamectin for flea control in naturally infested cats in West Central Florida, USA, Parasites and Vectors 11(1): 422.
- Favrot C, Rostaher A and Fischer N (2014). Clinical symptoms, diagnosis and therapy of feline allergic dermatitis, Schweizer Archiv für Tierheilkunde 156(7): 327-335.
- Forster-Van Hijfte MA, Curtis CF and White RN (1997). Resolution of exfoliative dermatitis and Malassezia pachydermatis overgrowth in a cat after surgical thymoma resection, Journal of Small Animal Practice 38(10): 451-454.
- Godfrey DR (1998). A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis, Journal of Small Animal Practice 39(8): 394-396.
- Hobi S, Linek M, Marignac G, Olivry T, Beco L, Nett C, Fontaine J, Roosje P, Bergvall K, Belova S, Koebrich S, Pin D, Kovalik M, Meury S, Wilhelm S and Favrot C (2011). Clinical characteristics and causes of pruritus in cats: a multicentre study on feline hypersensitivity-associated dermatoses, Veterinary Dermatology 22(5): 406-413.
- Jackson HA, Jackson MW, Coblentz L and Hammerberg B (2003). Evaluation of the clinical and allergen specific serum immunoglobulin E responses to oral challenge with cornstarch, corn, soy and a soy hydrolysate diet in dogs with spontaneous food allergy, Veterinary Dermatology 14(4): 181-187.
- Jones SM, Magnolfi CF, Cooke SK and Sampson HA (1995). Immunologic cross-reactivity among cereal grains and grasses in children with food hypersensitivity, Journal of Allergy and Clinical Immunology 96(3): 341-351.
- Maina E and Fontaine J (2019). Use of maropitant for the control of pruritus in non-flea, non-food-induced feline hypersensitivity dermatitis: an open-label, uncontrolled pilot study, Journal of Feline Medicine and Surgery 21(10): 967-972.
- Marsella R and De Benedetto A (2017). Atopic dermatitis in animals and people: an update and comparative review, Veterinary Sciences 4(3): 37.
- McClintock D, Austel M, Gogal RM Jr and Banovic F (2021). Oral dexamethasone sodium phosphate solution significantly reduces pruritus and clinical lesions in feline hypersensitivity dermatitis: an open-label study, Veterinary Dermatology 32(5): 497-e137.
- Noli C, Della Valle MF, Miolo A, Medori C, Schievano C and Skinalia Clinical Research Group (2019). Effect of dietary supplementation with ultramicronized palmitoylethanolamide in maintaining remission in cats with nonflea hypersensitivity dermatitis: a double-blind, multicentre, randomized, placebo-controlled study, Veterinary Dermatology 30(5): 387-e117.
- Nuttall T, Mueller R, Bensignor E, Verde M, Noli C, Schmidt V and Rème C (2009). Efficacy of a 0.0584% hydrocortisone aceponate spray in the management of canine atopic dermatitis: A randomised, double blind, placebo-controlled trial, Veterinary Dermatology 20(3): 191-198.
- Older CE, Diesel A, Patterson AP, Meason-Smith C, Johnson TJ, Mansell J, Suchodolski JS and Rodrigues Hoffman A (2017). The feline skin microbiota: the bacteria inhabiting the skin of healthy and allergic cats, Plos One 12(6): e0178555.
- Olivry T, Bexley J and Mougeot I (2017). Extensive protein hydrolyzation is indispensable to prevent IgE-mediated poultry allergen recognition in dogs and cats, BMC Veterinary Research 13(1): 251.
- Olivry T, Mueller RS and Prélaud P (2015). Critically appraised topic on adverse food reactions of companion animals (1): duration of elimination diets, BMC Veterinary Research 11: 225.
- Ravens PA, Xu BJ and Vogelnest LJ (2014). Feline atopic dermatitis: a retrospective study of 45 cases (2001-2012), Veterinary Dermatology 25(2): 95-102.
- Roberts ES, Speranza C, Friberg C, Griffin C, Steffan J, Roycroft L and King S (2016a). Confirmatory field study for the evaluation of ciclosporin at a target dose of 7.0 mg/kg (3.2 mg/lb) in the control of feline hypersensitivity dermatitis, Journal of Feline Medicine and Surgery 18(11): 889-897.
- Roberts ES, Tapp T, Trimmer A, Roycroft L and King S (2016b). Clinical efficacy and safety following dose tapering of ciclosporin in cats with hypersensitivity dermatitis, Journal of Feline Medicine and Surgery 18(11): 898-905.
- Santoro D, Pucheu-Haston CM, Prost C, Mueller RS and Jackson H (2021). Clinical signs and diagnosis of feline atopic syndrome: Detailed guidelines for a correct diagnosis, Veterinary Dermatology 32(1): 26-e6.
- Scarampella F, Abramo F and Noli C (2001). Clinical and histological evaluation of an analogue of palmitoylethanolamide, PLR 120 (comicronized Palmidrol INN) in cats with eosinophilic granuloma and eosinophilic plaque: a pilot study, Veterinary Dermatology 12(1): 29-39.
- Scott DW and Miller WH Jr (2013). Feline atopic dermatitis: a retrospective study of 194 cases (1988–2003), The Japanese Journal of Veterinary Dermatology 19(3): 135–147.
- Sierra P, Guillot J, Jacob H, Bussiéras S and Chermette R (2000). Fungal flora on cutaneous and mucosal surfaces of cats infected with feline immunodeficiency virus or feline leukemia virus, American Journal of Veterinary Research 61(2): 158-161.
- Thomas RC, Logas D, Radosta L and Harrison J (1999). Effects of a 1% hydrocortisone conditioner on haematological and biochemical parameters, adrenal function testing and cutaneous reactivity to histamine in normal and pruritic dogs, Veterinary Dermatology 10(2): 109-116.
- Volk AV, Belyavin CE, Varjonen K, Cadiergues M-C, Stevens KB and Bond R (2010). Malassezia pachydermatis and M. nana predominate amongst the cutaneous mycobiota of Sphynx cats, Journal of Feline Medicine and Surgery 12(12): 917-922.
- Williams H, Young DR, Qureshi T, Zoller H and Heckeroth AR (2014). Fluralaner, a novel isoxazoline, prevents flea (Ctenocephalides felis) reproduction in vitro and in a simulated home environment, Parasites and Vectors 7: 275.
