23 Sept 2025
Immune-mediated disease in dogs and cats: management and more
Kit Sturgess MA, VetMB, PhD, CertVR, DSAM, CertVC, FRCVS covers diagnosis and treatment, including with corticosteroids, of this issue.

Image: tmart_foto / Adobe Stock
The immune system plays a central role in the vast majority of disease processes that affect cats and dogs. It is a hugely complex system with many checks and balances designed to ensure the outcome for the individual is optimal; for example, appropriate recognition of danger signals and the right degree of response.
Not surprisingly, occasions exist where this system fails, many of which have underlying genetic drivers.
This article aims to focus on the management of immune-mediated disease (IMD) and, in particular, the options available for using conventional immunosuppressive drugs. IMD can be organ or whole organism focused, and is characterised by inflammation that is self-sustaining and associated with dysregulation of the immune response. IMD is often divided into an associated (secondary) group where an underlying condition has been identified that is presumed to be driving the immune dysregulation, such as haemolytic anaemia secondary to pancreatitis, and an unassociated (primary) group where no underlying cause has been identified.
The discussions in this article will assume that an appropriate investigation has been performed and that no treatable underlying association has been identified. When considering management of unassociated IMD (UIMD), as with any other management plan, it is essential to consider the whole patient to understand the impact of treatment on any pre-existing comorbidities.
Diagnosis of UIMD
Establishing a negative diagnosis is a real challenge – particularly as the best outcomes for the patient are usually associated with finding an underlying treatable cause, such as vector-borne infection.
- What tests should I perform?
- If my test gives a positive result, how will that affect the management of the patient (treatment, plan, prognosis)?
- What is my threshold for choosing whether to test (common versus uncommon versus rare underlying associations)?
- What are the risks and requirements of the investigation? Does the practice have the skills and equipment to undertake the tests planned?
- What are the costs of investigation? Is the cost-benefit association reasonable? Will the carer then have sufficient financial resource to treat the patient?
- What is the context? How possible will it be to treat the patient?
- Can the treatment plan be delivered (for example, an un-pillable cat)?
- What alternative management strategies are available, and what is the cost implication?
- How likely are significant complications to occur, and if they do, what additional treatment and costs may be required?
- What is the likely ability to sustain long-term treatment and monitoring, if needed?
Treatment of UIMD
Besides owner and patient factors, treatment choice will depend on a number of factors, including the condition being treated and how acute, progressive and life threatening it may be.
As IMD is associated with failure of immune regulation, a wide variety of drugs and nutraceuticals postulated to have immunomodulatory activity have been used as primary and adjunctive therapies. Robust evidence of efficacy in cats and dogs for many of them is lacking. In terms of treatment intensity, two options are available.
- Start with a lower end dose of a single therapy and monitor the patient response, increasing dose, changing or adding in additional therapies depending on the impact of the disease process. This approach is likely to take longer to resolve the clinical signs, but serious side effects are less likely, and it is more likely to lead to the optimal therapeutic combination. This approach is generally suited to slowly progressive or chronic disease.
- Start with aggressive, potentially high-dose multiple therapy and then, once the disease is under control, reduce the therapy slowly, observing for recurrence of signs. Clinical signs are likely to be controlled more quickly, but significant side effects of treatment are also more likely. Costs can be higher, as more intensive monitoring is generally required and drugs may be used that have not provided additional benefit. This approach is generally appropriate for patients with severe, potentially life-threatening or rapidly progressive disease.
Immunomodulators (biological response modifiers) can be divided into a number of broad categories.
- Drugs used primarily for their immunomodulatory activity.
- Conventional immunosuppressive drugs – prednisolone, ciclosporin, chlorambucil, or other chemotherapeutic agents (antimetabolites).
- Molecules that more specifically target certain parts of the immune system, often changing the cytokine environment or affect cellular signalling. A wide range of molecules, many of which are monoclonal antibodies, target various points along the inflammatory pathways associated with disease; for example, oclacitinib, a Janus kinase inhibitor used in the management of atopy; or infliximab, an inhibitor of tumour necrosis factor used to manage ulcerative colitis and Crohn’s disease.
- In people, helminths such as whipworm and hookworm are being investigated for their potential as highly effective treatments for the symptoms and/or disease process in disorders such as relapsing remitting multiple sclerosis, Crohn’s disease, allergies and asthma.
- Vaccines as immunostimulants.
- Intravenous immunoglobulin.
- Nutrients associated with immunomodulatory activity.
- Nutraceuticals such as Echinacea species and acemannan claim to boost the immune system. Some have similar potential problems associated with blanket immune stimulation, and side effects with Echinacea species use are reported in people and cats; it is not recommended for use in autoimmune disease in people.
- A number of pet food products also carry claims to support the immune system; these claims are based on their inclusion of omega-3 polyunsaturated fatty acids, vitamins and antioxidants. Zinc, for example, has effects on the immune system including an antifibrotic action and can be used in the management of inflammatory liver disease.
- Drugs that are used with the potential additional benefit of affecting the immune response beyond their primary use, such as doxycycline, metronidazole, cimetidine, levamisole, diethylcarbamazine or ursodeoxycholic acid.
- A wide variety of compounds have been used as vaccine adjuvants (for example, aluminium-based, saponins, oil, cytokines) – particularly in killed vaccines to boost their immunogenicity. They are undoubtedly effective, but also carry side-effect risks associated with vaccine reactions, most significantly injection site sarcoma.
- A number of homeopathic remedies claim to have activity to “boost” the immune system.
Use of conventional immunosuppressive drugs
If response to first-choice immunosuppressives is inadequate, then a second drug should be added.
If prednisolone is used as first choice then consensus opinion for second choices is to use ciclosporin, mycophenolate or azathioprine in dogs, and ciclosporin or chlorambucil in cats. Use of three immunosuppressive agents is not recommended, as the risk of fatal infections becomes significant and should be reserved for cases where different combinations of two agents have failed, the patient is deteriorating and an appropriate discussion with the carer has occurred.
Glucocorticoids
Glucocorticoids, most commonly prednisolone in the UK (prednisone in US), are used as they are an inexpensive and easily administered as tablets or liquid, although other options are available, and approximate dose equivalence is given in Table 1.
Where no specific contraindication to the use of glucocorticoids exists, such as heart failure or diabetes, it is the first choice immunosuppressive drug for most UIMD. Corticosteroids work at two levels, having both genomic and non-genomic effects to reduce B cell production of antibodies. Onset of action is rapid (hours); duration of action is listed in Table 1.
Corticosteroids have little effect on pre-existing antibodies, so it can take some time (seven to 10 days) for their full effect to become apparent. Specific acquired immunity tends to be affected less than non-specific immune responses.
Corticosteroids have a variety of effects, including:
- Decreased egress of inflammatory cells from the bloodstream into peripheral tissues.
- Decreased elaboration of inflammatory mediators, including prostaglandins and leukotrienes.
- Suppression of macrophage and neutrophil bactericidal functions.
- Decreased macrophage Fc receptor expression, with less effective phagocytosis.
- Decreased antigen presentation to T helper cells, with decreased T cell help for B cells.
- Decreased effector cell function, including that of natural killer cells.
- Inhibition of the passage of immune complexes through basement membranes.
- Antagonising the complement cascade.
- Masking the clinical signs of infection.
- Decreased number of mast cells, and suppressed histamine synthesis.
Many of these effects only occur at high or very high doses, and species differences in response exist.
Immunosuppressive doses of prednisolone in dogs and cats are in the region of 2mg/kg to 4mg/kg by mouth every 24 hours. At higher doses, and because in some individuals, duration of action may be as low as 12 hours, many clinicians will start at 1mg/kg to 2mg/kg given twice daily.
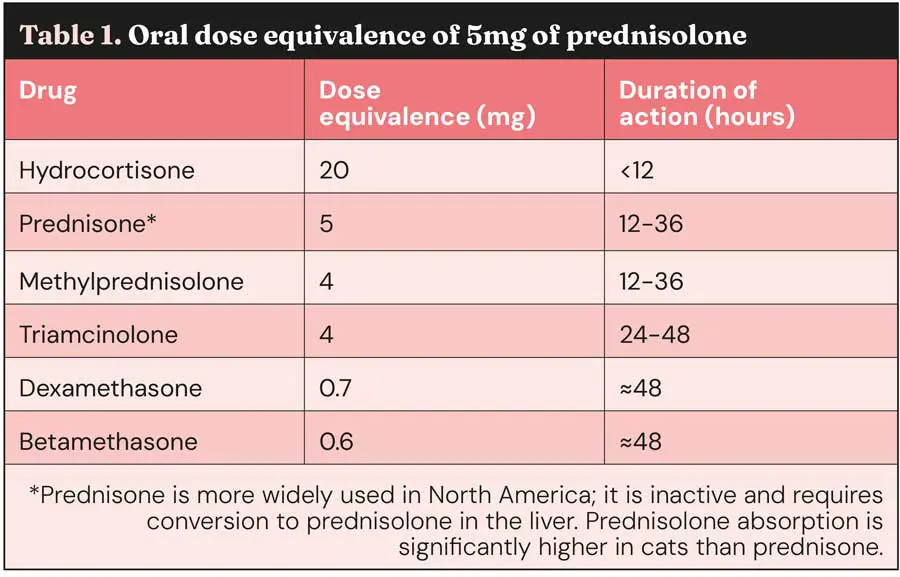
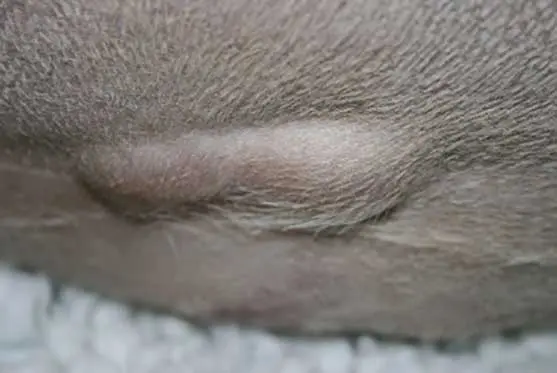
Limited evidence suggests that twice daily dosing is associated with a higher level of side effects. Like all drugs, dosing should be given on a body surface area rather than bodyweight, and prednisolone doses given at 2mg/kg in large and giant breed dogs are excessive and tend to cause significant and severe side effects (Figure 1), leading to a dose rate of 40mg/m2 to 50mg/m2 being used in dogs weighing more than 30kg to 35kg.
Although dose rates as high as 8mg/kg every 24 hours are suggested in cats, evidence to support this is non-existent. Indeed, no published evidence exists on the optimal dose of prednisolone for use as an immunosuppressive agent in dogs or cats, and this is likely to vary significantly between individuals.
Most dogs and cats on immunosuppressive doses of glucocorticoids will experience some side effects, acutely polyuria/polydipsia, polyphagia, excessive panting and behavioural changes/lethargy, chronically iatrogenic Cushingnoid signs and increased risk of urinary tract infections.
Other side effects are less common and would include anorexia and weight loss, bone resorption or inhibition of bone growth depending on age, calcinosis cutis, inhibition of collagen synthesis and weakening of surgical scars (Figure 1), ligaments and tendons, delayed wound healing, gastrointestinal (GI) side effects including diarrhoea with or without blood, ulceration that can progress to perforation, hepatopathy, hypertension, potassium loss, sodium and fluid retention, recrudescence of latent infections or decreased resistance to infection, and unmasking of diabetes mellitus.
Topical and locally active glucocorticoids are available, appropriate for some organ-specific immune-mediated diseases, have the advantage of limited systemic absorption (therefore, fewer side effects) and can potentially be used in patients where systemic glucocorticoids would be contraindicated. These include glucocorticoid-containing eye drops/ointments (note: do not penetrate beyond anterior chamber), topical dermatologic products such as hydrocortisone aceponate or in-ear preparations, inhaled glucocorticoids (fluticasone propionate is most widely used) and glucocorticoids formulated to act locally in the GI tract, such as budesonide.
Ciclosporin
For many, ciclosporin represents a good second-choice drug, as it is a veterinary licensed product. It is considered first-line treatment for perianal fistulas, keratoconjunctivitis sicca and some cases of atopic dermatitis.
Ciclosporin is a calcineurin inhibitor, functioning by binding to specific intracellular receptors and suppressing transcription of key cytokines involved in the innate and adaptive arms of the immune response.
Various studies have suggested that the calcineurin inhibitors also inhibit the number and/or function of regulatory T cells, which would work against attempts to correct the abnormal immune responses underlying autoimmune disease. Ciclosporin is lipophilic and hydrophobic, and must be solubilised before administration; micro-emulsified preparations are better absorbed than oil-based formulations. While the presence of food decreased the bioavailability of the micro-emulsified preparation of ciclosporin by 22% in dogs, it appeared to make no difference to clinical response in the treatment of canine atopic dermatitis; therefore, the usual recommendation to administer the drug two hours before or after feeding may be redundant in some clinical scenarios.
Although usually given orally, it is available as a topical ophthalmic ointment and an intravenous preparation, which can be considered in patients that are vomiting. Metabolism is primarily hepatic, with predominantly biliary excretion.
In dogs, average oral absorption of micro-emulsified ciclosporin is 36% and oil-based 20% to 25%. In cats, oral absorption is 25% to 29%; no difference in bioavailability has been found when drug administered to fed or fasted cats, or when mixed with food. For monitoring purposes, two-hour peak concentrations correlate better to overall exposure to ciclosporin than trough samples.
However, optimal blood concentration has not been objectively determined for any of the systemic immune-mediated disorders, and suggested levels have been based on extrapolation from the human literature, anecdotal evidence and experience gained in the setting of allotransplantation. Further, a lack of correlation exists between blood concentrations and clinical response. This is reflected in the veterinary literature; for example, suggested dose rates for dogs vary between 5mg/kg every 24 hours up to 10mg/kg every 12 hours. Serum monitoring is appropriate if a patient shows a lack of apparent response or potential toxicity.
Rarely, dogs have a ABCB1-1∆ mutation, leading to defective P-glycoprotein function, in which systemic accumulation of the ciclosporin may occur because of decreased biliary and renal excretion.
The major issues for many owners treating patients with ciclosporin are cost and palatability. Palatability can be a particular problem for cats, even using liquid preparations (100mg/ml) where dose volume is small. Ciclosporin tends not to be nephrotoxic or hepatotoxic in dogs and cats at the doses recommended in the veterinary literature, and is generally well tolerated in these species. The most common side effects reported in dogs are vomiting and diarrhoea, which may be reduced by freezing the capsules for 30 to 60 minutes and giving with a small amount of food.
Other commonly reported side effects include persistent otitis externa, urinary tract infections, anorexia, lymphadenopathy and lethargy. In cats, of the 2,506 adverse events reported to the US Food and Drug Administration, vomiting (n=559; 22%) and weight loss (n=349; 14%) were the most common.
Other relatively common side effects in approximate order of likelihood include retching, regurgitation, weight loss, diarrhoea, anorexia/hyporexia, lethargy/malaise and hypersalivation. Long-term ciclosporin therapy may also increase the risk of neoplasia in particular lymphoma – especially with concurrent prednisone or prednisolone therapy. Use of ciclosporin in diabetes is not advised due to the inhibition of insulin release and peripheral insulin resistance. Hirsutism may be observed in both dogs and cats.
Ciclosporin is available as an intravenous preparation that can be used in patients too sick to tolerate oral dosing. Suggested dose rates are 4mg/kg to 6mg/kg every 24 hours. Dose should be given over four hours (maximum dose 7.5mg/kg). Some clinicians will give chlorphenamine to reduce vesicant or anaphylactic impact, should it occur; however, limited minimal evidence suggests that this is an effective strategy, and a risk of intussusception is suggested.
Mycophenolate mofetil
Mycophenolate mofetil (MMF) is a prodrug that is hydrolysed by esterases present in the plasma, liver and kidneys to the active metabolite mycophenolic acid (MPA), a potent, reversible inhibitor of inosine monophosphate dehydrogenase necessary for the de novo synthesis of purine nucleotides.
T and B cell proliferation are inhibited; MMF also inhibits growth factor–induced smooth muscle and fibroblast proliferation, the maturation and function of dendritic cells, and the expression of various adhesion molecules required for homing of immune cells to areas of inflammation and intercellular crosstalk. MPA is highly protein bound and shows extensive enterohepatic recirculation. MMF is primarily metabolised via glucuronidation, but this is not the only mechanism, as conversion to MPA also occurs in cats.
MMF has been extensively used in dogs in a variety of immune-mediated diseases, immune-mediated anaemia, thrombocytopenia and polyarthritis, myasthenia gravis, meningoencephalomyelitis and immune-mediated glomerulonephritis. MMF has been much less used in cats, with case reports in cats with immune-mediated anaemia and polyarthritis.
Adverse effects in dogs include haemorrhagic diarrhoea, inflammatory diarrhoea, hepatotoxicity and myelosuppression. In cats, adverse effects include weight loss, loose stools, reduced food intake, hepatotoxicity and pancreatitis. Side effects seem dose dependent. Serial monitoring of haematology and biochemistry should be performed to detect bone marrow suppression, acute liver and kidney reactions. Reports, and the author’s experience, demonstrate that response is variable; comparative studies have not shown clear benefits over other agents such as ciclosporin, but fewer side effects were noted in dogs with immune-mediated thrombocytopenia treated with glucocorticoids and MMF compared to glucocorticoids and ciclosporin.
A starting dose of 10mg/kg by mouth every 24 hours for MMF is tolerated by most dogs and is the recommended starting dose in cats.
MMF is available as tablets and capsules (in sizes 250mg and 500mg), making it inconvenient for use in small dogs and cats; an oral suspension (200mg/ml) is available, but is expensive. A solution for infusion is also available, but has not been widely used in dogs and cats. Gastro-resistant tablets of MPA as mycophenolate sodium are also available, but their use is less researched, while does rates are about 75% of those for MMF.
Azathioprine
Azathioprine is toxic in cats and should not be used.
Azathioprine is a purine antagonist immunosuppressive; its onset of action is slow and may take up to six weeks to reach full efficacy. Azathioprine antagonises purine metabolism, thereby inhibiting RNA, DNA synthesis and mitosis. It may also cause chromosome breaks secondary to incorporation into nucleic acids and cellular metabolism may become disrupted by the drug’s ability to inhibit co-enzyme formation.
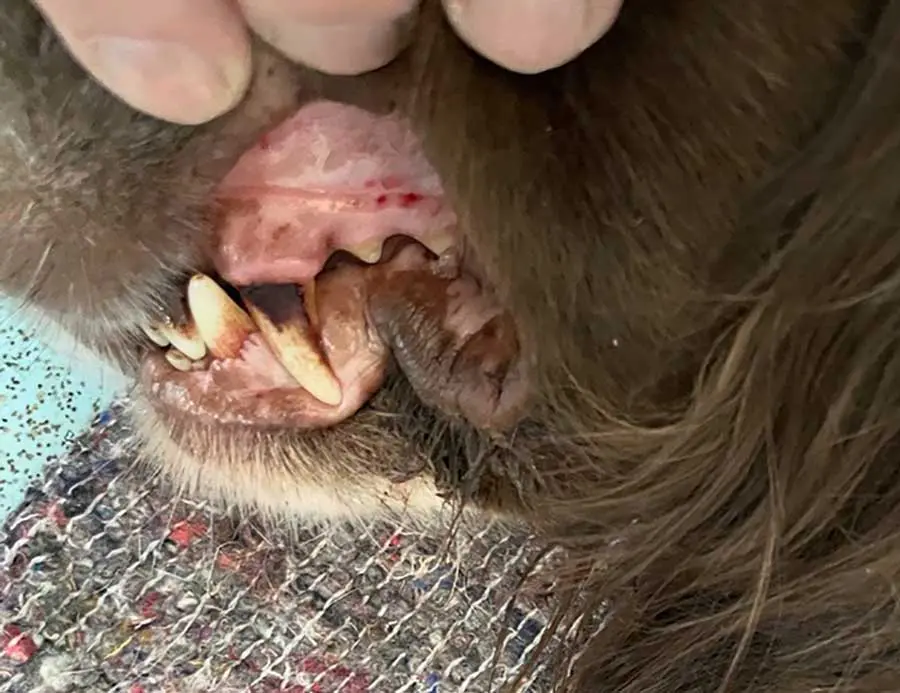
Azathioprine has greater activity on delayed hypersensitivity and cellular immunity than on humoral antibody responses. It is a known mutagen and teratogen, so is contraindicated in pregnancy and should be used with caution in patients with hepatic disease. Major side effects are bone marrow suppression (Figure 2), and GI effects including discomfort, anorexia and pancreatitis. Bone marrow suppression is usually reversible on withdrawal of the drug, but not in all patients.
Azathioprine is poorly absorbed from the GI tract and is rapidly metabolised to mercaptopurine. Mercaptopurine is rapidly taken up by lymphocytes and erythrocytes.
Cats have low activity of thiopurine methyltransferase (TPMT), one of the routes used to metabolise azathioprine. Dogs have variable TMPT activity levels (up to nine-fold variation) similar to that seen in humans, which may explain why some canine breeds and patients respond better and/or develop more myelotoxicity than others. TPMT activity is lower in the giant schnauzer and much higher in the Alaskan malamute than in other breeds. One study in dogs, however, did not show significant correlation between TMPT activity in red blood cells and drug toxicity (Rodriguez et al, 2004).
No prospective studies support any dosage protocol for azathioprine. It should also be noted that tablets should not be split. General recommendations are, initially, 2mg/kg by mouth once daily for one to four weeks, reducing to 0.5mg/kg to 2mg/kg every other day. Haematology should be monitored every seven to 14 days over the initial two months and periodically (every one to two months) thereafter.
Chlorambucil
Although a chemotherapeutic agent, chlorambucil is more widely used as an immunosuppressive agent in cats; in dogs, it is most commonly used in the management of protein-losing enteropathy.
Prospective clinical studies evaluating its use as an immunosuppressive agent are lacking. Chlorambucil is a cell cycle–nonspecific alkylating agent that shows high oral bioavailability and is highly plasma protein–bound, and rapidly metabolised in the liver to phenylacetic acid mustard, the principal active metabolite.
Chlorambucil is slow acting and of low toxicity, with myelosuppression being rare. Additional rare side effects may include hyporexia, vomiting and diarrhoea, generalised myoclonus, Fanconi syndrome and grand mal seizures, which have been observed in cats receiving high-dose pulse therapy.
Chlorambucil is generally administered without food, which interferes with its passive absorption. It has been primarily used in UMID in cats as an adjunctive therapy with corticosteroids.
A variety of dose rates have been suggested. Tablets are enteric coated and should not be subdivided. They should be stored at 2°C to 8°C. For many owners, the low frequency of dosing is an attractive option. For cats weighing more than 4kg, administer 2mg by mouth every 48 hours for two to four weeks and then reduce. For cats weighing less than 4kg, administer 2mg by mouth every 72 hours for two to four weeks and then reduce.
Alternatively, administer 20mg/m2 as a single dose every two weeks or 15mg/m2 for three consecutive days every three weeks. Periodic haematology monitoring should be considered.
For dogs, for the treatment of protein-losing enteropathy a dose rate of 2mg/m2 to 6mg/m2 by mouth every 24 hours is recommended.
Leflunomide
Leflunomide use in dogs is increasing, mainly because of the reduction in the cost of medication, its apparent efficacy in some conditions and relatively uncommon side effects. Leflunomide inhibits dihydroorotate dehydrogenase involved in pyrimidine synthesis, resulting in decreased T and B lymphocyte proliferation and antibody production.
Leflunomide also inhibits cytokine production and tyrosine kinase-mediated signal transduction, enhancing its immunosuppressive effect. Leflunomide is well absorbed orally, rapidly metabolised to teriflunomide (active drug) in the GI tract and liver, requiring cytochrome P450 enzyme metabolism; therefore, it should be used with caution in patients with hepatic disease or with drugs that affect cytochrome P450 enzyme metabolism, such as H2 blockers or azathioprine. Elimination is via renal and biliary excretion.
Common side effects are listed in Table 2. Most adverse effects have been mild and resolved upon discontinuation of therapy. Leflunomide has been used as a single agent to achieve remission in cases of immune-mediated polyarthritis and inflammatory colorectal polyps in dogs, and in combination for a variety of immune-mediated diseases including rheumatoid arthritis (with methotrexate) in cats and idiopathic polymyopathy in the vizsla.
Recent retrospective evaluation suggests a starting dosage of 2mg/kg every day, but higher dose rates have been used; much less research exists in cats.
Routine complete blood count and serum chemistry profile monitoring is recommended after two weeks, then every four to six weeks while administering leflunomide.
Conclusions
Prednisolone remains the primary drug used for most cases of UIMD. A few occasions exist where other drugs are used as primary therapy; they are most commonly used in conjunction with prednisolone as a way of sparing steroid side effects or where prednisolone alone appears ineffective.
The choice of a second immunosuppressive agent is poorly evidenced and should be based on individual patient circumstance, clinician experience, ability to monitor response and side effects, ans cost and ability to administer the drug. Table 2 gives suggested dose rates for these immunosuppressive agents.
No specific studies look at the timing of introducing a second agent, but most require a number of days or weeks to reach clinical effect, so in those cases that are not responding well to first-choice therapy, the general recommendation is to introduce a second agent sooner rather than later.
Equally, no studies look at how to withdraw therapy to see whether the UIMD is showing a durable response.
Where a combination of drugs is being used, the author will decrease the drug that is associated with most side effects first and then begin to reduce the second drug, often alternating drug dose reduction.
Depending on the patient, dose reduction can either be in administered dose or frequency of dosing.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
- This article appeared in Vet Times (2025), Volume 55, Issue 38, Pages 6-12
Companion animal veterinary studies and findings: August 2025 – including latest study on immune-mediated polyarthritis
Author
Kit Sturgess graduated from the University of Cambridge in 1986, then spent six years in general veterinary practice. He has further professional qualifications in imaging, cardiology and internal medicine, as well as a PhD for looking at the effects of FIV on mucosal immune function. Kit is a fellow of the RCVS, a specialist in small animal medicine and an advanced practitioner in veterinary cardiology. He has been seeing referral small animal medicine cases for the past 25 years – both at university-based and private specialist practices. Kit’s love of teaching and learning led him to develop a new, more flexible, role combining lecturing, writing and clinic time. The majority of his clinical time is spent providing an internal medicine service at Optivet Referrals in Havant, Hampshire. Kit maintains a keen interest in many areas of internal medicine and has authored numerous articles and two textbooks, and presented lectures and research abstracts at conferences worldwide.
References
- Bacek LM and Macintire DK (2011). Treatment of primary immune-mediated hemolytic anemia with mycophenolate mofetil in two cats, J Vet Emerg Crit Care 21(1): 45-49.
- Barnoon I et al (2016). Retrospective evaluation of combined mycophenolate mofetil and prednisone treatment for meningoencephalomyelitis of unknown etiology in dogs: 25 cases (2005-2011), J Vet Emerg Crit Care 26(1): 116-124.
- Cummings FO and Rizzo SA (2017). Treatment of presumptive primary immune-mediated thrombocytopenia with mycophenolate mofetil versus cyclosporine in dogs, J Small Anim Pract 58(2): 96-102.
- LeVine DN et al (2024). ACVIM consensus statement on the treatment of immune thrombocytopenia in dogs and cats, J Vet Intern Med 38(4): 1,982-2,007.
- Rodriguez DB et al (2004). Relationship between red blood cell thiopurine methyltransferase activity and myelotoxicity in dogs receiving azathioprine, J Vet Intern Med 18(3): 339-345.
- Swann JW et al (2019). ACVIM consensus statement on the treatment of immune-mediated hemolytic anemia in dogs, J Vet Intern Med 33(3): 1,141-1,172.
- Viviano KR (2022). Glucocorticoids, cyclosporine, azathioprine, chlorambucil, and mycophenolate in dogs and cats: clinical uses, pharmacology, and side effects, Vet Clin North Am Small Anim Pract 52(3): 797-817.
